Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy
- PMID: 32801698
- PMCID: PMC7398757
- DOI: 10.2147/IJN.S256898
Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy
Abstract
Background: Human epidermal growth factor receptor2 (Her2) positive breast cancer represents 25% of breast cancer cases. Targeted therapy with Her2 monoclonal antibody, trastuzumab (TZ), represents the first-line treatment for this type of breast cancer. In addition, neratinib, an irreversible inhibitor of the HER-2 receptor tyrosine kinase, has recently been approved as adjuvant therapy to TZ. This study aims to formulate (TZ)-grafted dendrimers loaded with neratinib, allowing a dual treatment alongside reducing the associated resistance as well as targeted therapy.
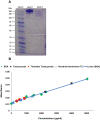
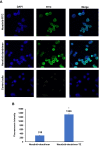
Methods: TZ was conjugated on the surface of dendrimer using hetero-cross linker, MAL-PEG-NHS, and the zeta potential, and in vitro release of neratinib from dendrimers was characterized. Formulated dendrimers were also fluorescently conjugated with fluorescein isothiocyanate to visualize and quantify their SKBR-3 cellular uptake.
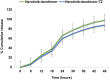
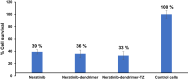
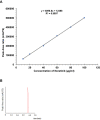
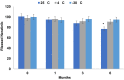
Results: The G4 PAMAM dendrimer showed successful encapsulation of neratinib and a sustained release profile. Comparative in vitro studies revealed that these TZ-targeted dendrimers loaded with neratinib were more selective and have higher antiproliferation activity against SKBR-3 cells compared to neratinib alone and neratinib loaded dendrimer.
Conclusion: In the current study, neratinib loaded in plain and trastuzumab-grafted dendrimer were successfully prepared. Enhanced cellular uptake of trastuzumab conjugated dendrimers was shown, together with a higher cytotoxic effect than plain neratinib dendrimers. These findings suggest the potential of TZ-conjugated dendrimers as targeting carrier for cytotoxic drugs, including neratinib.
Keywords: Her2-positive; breast cancer; neratinib; targeted dendrimer; trastuzumab.
© 2020 Aleanizy et al.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


References
-
- Stewart B, Wild CP. World Cancer Report 2014. lyon, france: International agency for research on cancer. World Health Organization. 2014; 630.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

