NOD1/2 and the C-Type Lectin Receptors Dectin-1 and Mincle Synergistically Enhance Proinflammatory Reactions Both In Vitro and In Vivo
- PMID: 32801829
- PMCID: PMC7383029
- DOI: 10.2147/JIR.S245638
NOD1/2 and the C-Type Lectin Receptors Dectin-1 and Mincle Synergistically Enhance Proinflammatory Reactions Both In Vitro and In Vivo
Abstract
Purpose: Pathogens consist of a wide variety of evolutionarily conserved molecular structures that are recognized by pattern recognition receptors (PRRs) of innate immunity. Reasonably assuming that no single PRR is ever likely to be the sole trigger of the immune response during infection, a great deal remains unknown about collaborative mechanisms and consequential crosstalk effects between multiple PRRs belonging to different families. Here, we aimed to investigate inflammatory response to combined stimulation of cytosolic nucleotide-binding oligomerization domain (NOD) receptors: NOD1, NOD2 and membrane-bound C-type lectin receptors (CLRs): Mincle and Dectin-1 in comparison to individual stimulation both in vitro and in vivo.
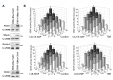
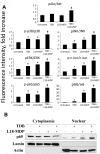
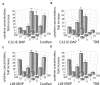
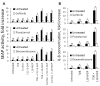
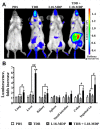
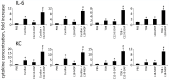
Materials and methods: For in vitro studies, we used human monocytic THP-1 cells endogenously expressing NOD1,2, as well as Mincle and Dectin-1 receptors. Using reporter gene and immunoassay approaches, we measured activity of key proinflammatory transcription factors (NF-κB and AP-1) and cytokine production after addition of specific PRR agonists or their pairwise combinations. In vivo NF-κB activity (bioluminescent detection in NF-κB-Luc transgenic mice), as well as cytokine levels in mouse blood serum, was measured 3 hours after intramuscular injection of PRR agonists.
Results: We detected that combined stimulation of NOD1/2 and C-type lectin receptors (Dectin-1, Mincle) strongly potentiates NF-κB and AP-1 transcription factor activity in human monocytic THP-1 cells, as well as resulting in enhanced levels of IL-8 cytokine production. We demonstrated that RIP2- and Syk-dependent signaling pathways downstream of NOD1/2 and Dectin-1/Mincle, respectively, are essential for the potentiated proinflammatory cell response. Lastly, we confirmed that synergy between NOD and C-type lectin receptors resulting in potentiated levels of NF-κB activation and cytokine (IL-6, KC) production also occurs in vivo.
Conclusion: These findings originally indicate cooperation between NODs and CLRs, leading to potentiated levels of proinflammatory immune response both in vitro and in vivo.
Keywords: collaboration; innate immunity; pattern recognition receptors; synergy.
© 2020 Tukhvatulin et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding this manuscript.
Figures
Similar articles
-
C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation.J Biol Chem. 2014 Oct 24;289(43):30052-62. doi: 10.1074/jbc.M114.588574. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202022 Free PMC article.
-
Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to Salmonella enterica serovar Typhimurium infection.Infect Immun. 2013 Oct;81(10):3855-64. doi: 10.1128/IAI.00525-13. Epub 2013 Jul 29. Infect Immun. 2013. PMID: 23897616 Free PMC article.
-
Activation of NF-κB and respiratory burst following Aspergillus fumigatus stimulation of macrophages.Immunobiology. 2014 Jan;219(1):25-36. doi: 10.1016/j.imbio.2013.06.013. Epub 2013 Jul 4. Immunobiology. 2014. PMID: 23886693
-
C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses.Cell Mol Immunol. 2012 Mar;9(2):105-12. doi: 10.1038/cmi.2011.58. Epub 2012 Jan 16. Cell Mol Immunol. 2012. PMID: 22246129 Free PMC article. Review.
-
Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications.J Leukoc Biol. 2019 Apr;105(4):669-680. doi: 10.1002/JLB.2RU0718-290R. Epub 2018 Dec 5. J Leukoc Biol. 2019. PMID: 30517768 Review.
Cited by
-
Chemotherapeutic Drugs Induce Different Gut Microbiota Disorder Pattern and NOD/RIP2/NF-κB Signaling Pathway Activation That Lead to Different Degrees of Intestinal Injury.Microbiol Spectr. 2022 Dec 21;10(6):e0167722. doi: 10.1128/spectrum.01677-22. Epub 2022 Oct 12. Microbiol Spectr. 2022. PMID: 36222691 Free PMC article.
-
Effects of a β-Glucan-Rich Blend of Medicinal Mushrooms and Botanicals on Innate Immune Cell Activation and Function Are Enhanced by a Very Low Dose of Bovine Colostrum Peptides.Molecules. 2024 Jun 12;29(12):2787. doi: 10.3390/molecules29122787. Molecules. 2024. PMID: 38930852 Free PMC article.
References
-
- Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukocyte Biol. 2006;80(2):267–277. doi:10.1189/jlb.1105626 - DOI - PubMed
-
- Tukhvatulin AI, Gitlin II, Shcheblyakov DV, et al. Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to salmonella enterica serovar typhimurium infection. Infect Immun. 2013;81(10):3855–3864. doi:10.1128/IAI.00525-13 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

