SRF Potentiates Colon Cancer Metastasis and Progression in a microRNA-214/PTK6-Dependent Manner
- PMID: 32801887
- PMCID: PMC7395694
- DOI: 10.2147/CMAR.S257422
SRF Potentiates Colon Cancer Metastasis and Progression in a microRNA-214/PTK6-Dependent Manner
Retraction in
-
SRF Potentiates Colon Cancer Metastasis and Progression in a microRNA-214/PTK6-Dependent Manner [Retraction].Cancer Manag Res. 2022 Sep 23;14:2869-2870. doi: 10.2147/CMAR.S390685. eCollection 2022. Cancer Manag Res. 2022. PMID: 36176695 Free PMC article.
Abstract
Objective: Serum response factor (SRF), a sequence-specific transcription factor, is closely related to metastasis of gastric cancer, a digestive tract cancer. Herein, we probed the effect of SRF on metastasis and progression of colon cancer (CC), another digestive tract disorder, and the detailed mechanism.
Methods: Microarray analysis was conducted on tumor and adjacent tissues to filter differentially expressed miRNA, followed by RT-qPCR validation in CC cell lines. The transcription factor and the target gene of microRNA-214 (miR-214) were predicted, and their binding relationships were tested by luciferase reporter assays and ChIP assays. Subsequently, SRF and protein tyrosine kinase 6 (PTK6) expression in CC patients and cells was evaluated by RT-qPCR, while JAK2 and STAT3 expression in cells by Western blot analysis. To further explore functions of miR-214, PTK6 and SRF on CC, CC cells were delivered with si-PTK6, miR-214 mimic and/or SRF overexpression.
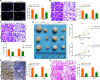
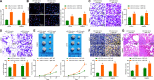
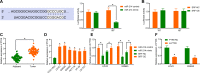
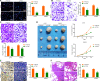
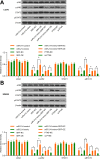
Results: miR-214 expressed poorly in CC tissues and cell lines, which related to advanced TNM staging and survival. miR-214 mimic inhibited proliferation, migration, invasion, xenograft tumor growth and metastasis of CC cells. SRF, overexpressed in CC samples and cells, suppressed the transcription of miR-214. Meanwhile, SRF upregulation counteracted the inhibitory role of miR-214 mimic in CC cell growth. miR-214 negatively regulated PTK6 expression to impair the JAK2/STAT3 pathway activation, thereby halting CC cell proliferation, migration, invasion, xenograft tumor growth and metastasis.
Conclusion: Altogether, miR-214 may perform as a tumor suppressor in CC, and the SRF/miR-214/PTK6/JAK2/STAT3 axis could be applied as a biomarker and potential therapeutic target.
Keywords: Colon cancer; PTK6; SRF; The JAK2/STAT3 pathway; microRNA-214.
© 2020 Li et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
MicroRNA-363 inhibits angiogenesis, proliferation, invasion, and migration of renal cell carcinoma via inactivation of the Janus tyrosine kinases 2-signal transducers and activators of transcription 3 axis by suppressing growth hormone receptor gene.J Cell Physiol. 2019 Mar;234(3):2581-2592. doi: 10.1002/jcp.27020. Epub 2018 Sep 19. J Cell Physiol. 2019. Retraction in: J Cell Physiol. 2022 Apr;237(4):2294. doi: 10.1002/jcp.30633. PMID: 30229899 Retracted.
-
MicroRNA-375 reverses the expression of PD-L1 by inactivating the JAK2/STAT3 signaling pathways in gastric cancer.Clin Res Hepatol Gastroenterol. 2021 Sep;45(5):101574. doi: 10.1016/j.clinre.2020.10.015. Epub 2020 Dec 4. Clin Res Hepatol Gastroenterol. 2021. PMID: 33272890
-
In vivo and in vitro effects of microRNA-221 on hepatocellular carcinoma development and progression through the JAK-STAT3 signaling pathway by targeting SOCS3.J Cell Physiol. 2019 Apr;234(4):3500-3514. doi: 10.1002/jcp.26863. Epub 2018 Oct 28. J Cell Physiol. 2019. Retraction in: J Cell Physiol. 2022 Mar;237(3):2000. doi: 10.1002/jcp.30512. PMID: 30370582 Retracted.
-
MicroRNA‑7 suppresses human colon cancer invasion and proliferation by targeting the expression of focal adhesion kinase.Mol Med Rep. 2016 Feb;13(2):1297-303. doi: 10.3892/mmr.2015.4643. Epub 2015 Dec 4. Mol Med Rep. 2016. PMID: 26648422
-
Therapeutic Potential of Protein Tyrosine Kinase 6 in Colorectal Cancer.Cancers (Basel). 2023 Jul 21;15(14):3703. doi: 10.3390/cancers15143703. Cancers (Basel). 2023. PMID: 37509364 Free PMC article. Review.
Cited by
-
miR-423-5p Regulates Skeletal Muscle Growth and Development by Negatively Inhibiting Target Gene SRF.Genes (Basel). 2024 May 10;15(5):606. doi: 10.3390/genes15050606. Genes (Basel). 2024. PMID: 38790235 Free PMC article.
-
Knockdown of microRNA-214-3p Promotes Tumor Growth and Epithelial-Mesenchymal Transition in Prostate Cancer.Cancers (Basel). 2021 Nov 23;13(23):5875. doi: 10.3390/cancers13235875. Cancers (Basel). 2021. PMID: 34884984 Free PMC article.
-
KIAA1199 Correlates With Tumor Microenvironment and Immune Infiltration in Lung Adenocarcinoma as a Potential Prognostic Biomarker.Pathol Oncol Res. 2022 Nov 7;28:1610754. doi: 10.3389/pore.2022.1610754. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36419650 Free PMC article.
-
Interference of PTK6/GAB1 signaling inhibits cell proliferation, invasion, and migration of cervical cancer cells.Mol Med Rep. 2022 Sep;26(3):284. doi: 10.3892/mmr.2022.12800. Epub 2022 Jul 27. Mol Med Rep. 2022. PMID: 35894144 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

