Osmolyte-Induced Folding and Stability of Proteins: Concepts and Characterization
- PMID: 32802087
- PMCID: PMC7393045
- DOI: 10.22037/ijpr.2020.112621.13857
Osmolyte-Induced Folding and Stability of Proteins: Concepts and Characterization
Abstract
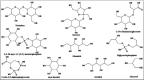
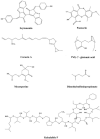
It is well-known that the typical protein's three-dimensional structure is relatively unstable in harsh conditions. A practical approach to maintain the folded state and thus improve the stability and activity of proteins in unusual circumstances is to directly apply stabilizing substances such as osmolytes to the protein-containing solutions. Osmolytes as natural occurring organic molecules typically called "compatible" solutes, based on the concept that they do not perturb cellular components. However, urea and guanidine hydrochloride (GuHCl) as denaturing osmolytes destabilize many macromolecular structures and inhibit functions. Several studies have been so far performed to explain the actual interaction of an osmolyte with a protein. The present review is aimed to achieve a collective knowledge of the progress arise in the field of osmolyte-protein interactions. The following is also an overview of the main techniques to measure protein stability in the presence of osmolytes.
Keywords: Folding state; Osmolyte; Preferential hydration; Protein stability; Protein structure.
Figures



References
-
- Harano Y, Masahiro K. On the physics of pressure denaturation of proteins. J. Phys. Condens. Matter. 2006;7:L107.
-
- Yoshioka S, Kelly MF, Yukio A, Michael JP. Effect of sugars on the molecular motion of freeze-dried protein formulations reflected by NMR relaxation times. Pharm. Res. . 2011;12:3237–47. - PubMed
-
- Neurath H, Greenstein JP, Putnam FW, Erickson JA. The chemistry of protein denaturation. Chem. Rev. 1944;5:157–265.
-
- Gruebele M. The fast protein folding problem. Annu. Rev. Phys. Chem. 1999;1:485–516. - PubMed
-
- Pande VS, Grosberg AY, Tanaka T. Heteropolymer freezing and design: towards physical models of protein folding. Rev. Mod. Phys. 2000;1:259–86.
Publication types
LinkOut - more resources
Full Text Sources
