Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins
- PMID: 32802145
- PMCID: PMC7425807
- DOI: 10.1016/j.nantod.2020.100898
Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins
Abstract
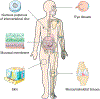
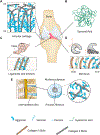
Negatively charged tissues are ubiquitous in the human body and are associated with a number of common diseases yet remain an outstanding challenge for targeted drug delivery. While the anionic proteoglycans are critical for tissue structure and function, they make tissue matrix dense, conferring a high negative fixed charge density (FCD) that makes drug penetration through the tissue deep zones and drug delivery to resident cells extremely challenging. The high negative FCD of these tissues is now being utilized by taking advantage of electrostatic interactions to create positively charged multi-stage delivery methods that can sequentially penetrate through the full thickness of tissues, create a drug depot and target cells. After decades of work on attempting delivery using strong binding interactions, significant advances have recently been made using weak and reversible electrostatic interactions, a characteristic now considered essential to drug penetration and retention in negatively charged tissues. Here we discuss these advances using examples of negatively charged tissues (cartilage, meniscus, tendons and ligaments, nucleus pulposus, vitreous of eye, mucin, skin), and delve into how each of their structures, tissue matrix compositions and high negative FCDs create barriers to drug entry and explore how charge interactions are being used to overcome these barriers. We review work on tissue targeting cationic peptide and protein-based drug delivery, compare and contrast drug delivery designs, and also present examples of technologies that are entering clinical trials. We also present strategies on further enhancing drug retention within diseased tissues of lower FCD by using synergistic effects of short-range binding interactions like hydrophobic and H-bonds that stabilize long-range charge interactions. As electrostatic interactions are incorporated into design of drug delivery materials and used as a strategy to create properties that are reversible, tunable and dynamic, bio-electroceuticals are becoming an exciting new direction of research and clinical work.
Keywords: Cationic drug carriers; Cationic protein drug carriers; Cell penetrating peptides; Electro-diffusive transport; Electrostatic charge interactions; Negatively charged tissues; Targeted drug delivery.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
