Lentinan-functionalized Selenium Nanoparticles target Tumor Cell Mitochondria via TLR4/TRAF3/MFN1 pathway
- PMID: 32802180
- PMCID: PMC7415812
- DOI: 10.7150/thno.46467
Lentinan-functionalized Selenium Nanoparticles target Tumor Cell Mitochondria via TLR4/TRAF3/MFN1 pathway
Erratum in
-
Erratum: Lentinan-functionalized Selenium Nanoparticles target Tumor Cell Mitochondria via TLR4/TRAF3/MFN1 pathway: Erratum.Theranostics. 2022 Nov 2;12(17):7645. doi: 10.7150/thno.78949. eCollection 2022. Theranostics. 2022. PMID: 36438475 Free PMC article.
Abstract
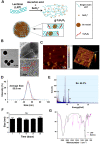
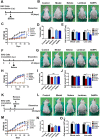
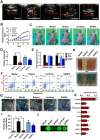
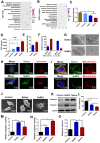
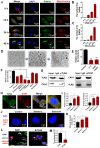
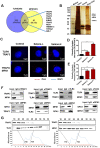
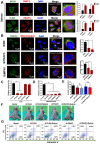
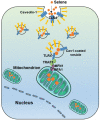
Rationale: Malignant ascites caused by cancer cells results in poor prognosis and short average survival time. No effective treatment is currently available for malignant ascites. In this study, the effects of lentinan (LNT)-functionalized selenium nanoparticles (Selene) on malignant ascites were evaluated. Furthermore, the mechanism of Selene targeting mitochondria of tumor cells were also investigated. Methods: Selene were synthesized and characterized by TEM, AFM and particle size analysis. The OVCAR-3 and EAC cells induced ascites models were used to evaluate the effects of Selene on malignant ascites. Proteomic analysis, immunofluorescence, TEM and ICP-MS were used to determine the location of Selene in tumor cells. Mitochondrial membrane potential, ROS, ATP content, and caspase-1/3 activity were detected to evaluate the effect of Selene on mitochondrial function and cell apoptosis. Immunofluorescence, Co-IP, pull-down, duolink, Western blot, and FPLC were used to investigate the pathway of Selene targeting mitochondria. Results: Selene could effectively inhibit ascites induced by OVCAR-3 and EAC cells. Selene was mainly located in the mitochondria of tumor cells and induced apoptosis of tumor cells. The LNT in Selene was involved in caveolae-mediated endocytosis through the interaction between toll-like receptor-4 (TLR4) and caveolin 1 (CAV1). Furthermore, the Selene in the endocytic vesicles could enter the mitochondria via the mitochondrial membrane fusion pathway, which was mediated by TLR4/TNF receptor associated factor 3 (TRAF3)/mitofusin-1 (MFN1) protein complex. Conclusion: Selene is a candidate anticancer drug for the treatment of malignant ascites. And TLR4/TRAF3/MFN1 may be a specific nano-drug delivery pathway that could target the mitochondria.
Keywords: lentinan; malignant ascites; mitochondria targeting pathway; ovarian cancer; selenium nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity.Carbohydr Polym. 2015 Mar 6;117:434-442. doi: 10.1016/j.carbpol.2014.09.088. Epub 2014 Oct 12. Carbohydr Polym. 2015. PMID: 25498656
-
Mitofusin 1 degradation is induced by a disruptor of mitochondrial calcium homeostasis, CGP37157: a role in apoptosis in prostate cancer cells.Int J Oncol. 2014 May;44(5):1767-73. doi: 10.3892/ijo.2014.2343. Epub 2014 Mar 13. Int J Oncol. 2014. PMID: 24626641
-
Dynamin-related protein 1-mediated mitochondrial fission contributes to IR-783-induced apoptosis in human breast cancer cells.J Cell Mol Med. 2018 Sep;22(9):4474-4485. doi: 10.1111/jcmm.13749. Epub 2018 Jul 11. J Cell Mol Med. 2018. PMID: 29993201 Free PMC article.
-
Targeting mitochondria in fighting cancer.Curr Pharm Des. 2011 Dec 1;17(36):4034-46. doi: 10.2174/138161211798764933. Curr Pharm Des. 2011. PMID: 22188453 Review.
-
Cross-Talk Between Selenium Nanoparticles and Cancer Treatment Through Autophagy.Biol Trace Elem Res. 2024 Jul;202(7):2931-2940. doi: 10.1007/s12011-023-03886-8. Epub 2023 Oct 10. Biol Trace Elem Res. 2024. PMID: 37817045 Review.
Cited by
-
Selenium Nanoparticles Stabilized by β-Glucan Nanotubes from Black Fungus and Their Effects on the Proliferation, Apoptosis, and Cell Cycle of HepG2 Cells.ACS Omega. 2023 Oct 30;8(48):45358-45368. doi: 10.1021/acsomega.3c04244. eCollection 2023 Dec 5. ACS Omega. 2023. PMID: 38075754 Free PMC article.
-
Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine.Nanomaterials (Basel). 2023 Jan 19;13(3):424. doi: 10.3390/nano13030424. Nanomaterials (Basel). 2023. PMID: 36770385 Free PMC article. Review.
-
Selenium-Containing Compound Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Regulating the MAPK/AP-1 Pathway.Inflammation. 2021 Dec;44(6):2518-2530. doi: 10.1007/s10753-021-01521-z. Epub 2021 Sep 6. Inflammation. 2021. PMID: 34487287
-
Selenium nanoparticles attenuate retinal pathological angiogenesis by disrupting cell cycle distribution.Nanomedicine (Lond). 2025 Apr;20(8):803-816. doi: 10.1080/17435889.2025.2480046. Epub 2025 Mar 21. Nanomedicine (Lond). 2025. PMID: 40114604
-
Mechanisms of action of fungal polysaccharides and their therapeutic effect.Eur J Clin Nutr. 2025 May;79(5):383-396. doi: 10.1038/s41430-024-01527-4. Epub 2024 Oct 21. Eur J Clin Nutr. 2025. PMID: 39433857 Review.
References
-
- Tsikouras P, Tsagias N, Pinidis P, Csorba R, Vrachnis N, Dafopoulos A. et al. The contribution of catumaxomab in the treatment of malignant ascites in patients with ovarian cancer: a review of the literature. Arch Gynecol Obstet. 2013;288:581–5. - PubMed
-
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

