Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration
- PMID: 32802181
- PMCID: PMC7415811
- DOI: 10.7150/thno.44281
Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration
Abstract
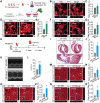
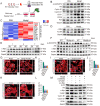
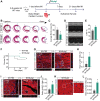
Myeloid-derived growth factor (Mydgf), a paracrine protein secreted by bone marrow-derived monocytes and macrophages, was found to protect against cardiac injury following myocardial infarction (MI) in adult mice. We speculated that Mydgf might improve heart function via myocardial regeneration, which is essential for discovering the target to reverse heart failure. Methods: Two genetic mouse lines were used: global Mydgf knockout (Mydgf-KO) and Mydgf-EGFP mice. Two models of cardiac injury, apical resection was performed in neonatal and MI was performed in adult mice. Quantitative reverse transcription-polymerase chain reaction, western blot and flow cytometry were performed to study the protein expression. Immunofluorescence was performed to detect the proliferation of cardiomyocytes. Heart regeneration and cardiac function were evaluated by Masson's staining and echocardiography, respectively. RNA sequencing was employed to identify the key involved in Mydgf-induced cardiomyocyte proliferation. Mydgf recombinant protein injection was performed as a therapy for cardiac repair post MI in adult mice. Results: Mydgf expression could be significantly induced in neonatal mouse hearts after cardiac injury. Unexpectedly, we found that Mydgf was predominantly expressed by endothelial cells rather than macrophages in injured neonatal hearts. Mydgf deficiency impeded neonatal heart regeneration and injury-induced cardiomyocyte proliferation. Mydgf recombinant protein promoted primary mouse cardiomyocyte proliferation. Employing RNA sequencing and functional verification, we demonstrated that c-Myc/FoxM1 pathway mediated Mydgf-induced cardiomyocyte expansion. Mydgf recombinant protein improved cardiac function in adult mice after MI injury with inducing cardiomyocyte proliferation. Conclusion: Mydgf promotes cardiomyocyte proliferation by activating c-Myc/FoxM1 pathway and improves heart regeneration both in neonatal and adult mice after cardiac injury, providing a potential target to reverse cardiac remodeling and heart failure.
Keywords: FoxM1; Mydgf; c-Myc; cardiomyocyte proliferation; heart regeneration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
gp130 Controls Cardiomyocyte Proliferation and Heart Regeneration.Circulation. 2020 Sep 8;142(10):967-982. doi: 10.1161/CIRCULATIONAHA.119.044484. Epub 2020 Jun 30. Circulation. 2020. PMID: 32600062
-
Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction.Nat Med. 2015 Feb;21(2):140-9. doi: 10.1038/nm.3778. Epub 2015 Jan 12. Nat Med. 2015. PMID: 25581518
-
Long Noncoding RNA CPR (Cardiomyocyte Proliferation Regulator) Regulates Cardiomyocyte Proliferation and Cardiac Repair.Circulation. 2019 Jun 4;139(23):2668-2684. doi: 10.1161/CIRCULATIONAHA.118.035832. Epub 2019 Mar 5. Circulation. 2019. PMID: 30832495
-
Cell Cycle-Mediated Cardiac Regeneration in the Mouse Heart.Curr Cardiol Rep. 2019 Sep 16;21(10):131. doi: 10.1007/s11886-019-1206-9. Curr Cardiol Rep. 2019. PMID: 31529165 Free PMC article. Review.
-
Neonatal injury models: integral tools to decipher the molecular basis of cardiac regeneration.Basic Res Cardiol. 2022 May 3;117(1):26. doi: 10.1007/s00395-022-00931-w. Basic Res Cardiol. 2022. PMID: 35503383 Free PMC article. Review.
Cited by
-
Identification of myeloid-derived growth factor as a mechanically-induced, growth-promoting angiocrine signal for human hepatocytes.Nat Commun. 2024 Feb 5;15(1):1076. doi: 10.1038/s41467-024-44760-y. Nat Commun. 2024. PMID: 38316785 Free PMC article.
-
Myeloid-derived growth factor regulates neutrophil motility in interstitial tissue damage.J Cell Biol. 2021 Aug 2;220(8):e202103054. doi: 10.1083/jcb.202103054. Epub 2021 May 28. J Cell Biol. 2021. PMID: 34047769 Free PMC article.
-
The Growth Factors: Potential Biomarkers and Therapeutic Targets in Kidney Diseases.Kidney Dis (Basel). 2022 Sep 12;8(5):368-380. doi: 10.1159/000526208. eCollection 2022 Nov. Kidney Dis (Basel). 2022. PMID: 36466071 Free PMC article. Review.
-
The Clinical Relevance and Tumor Promoting Function of C19orf10 in Kidney Renal Clear Cell Carcinoma.Front Oncol. 2021 Sep 6;11:725959. doi: 10.3389/fonc.2021.725959. eCollection 2021. Front Oncol. 2021. PMID: 34552877 Free PMC article.
-
Cardiomyocyte S1PR1 promotes cardiac regeneration via AKT/mTORC1 signaling pathway.Theranostics. 2025 Jan 2;15(4):1524-1551. doi: 10.7150/thno.103797. eCollection 2025. Theranostics. 2025. PMID: 39816679 Free PMC article.
References
-
- Ponnusamy M, Liu F, Zhang YH, Li RB, Zhai M, Liu F. et al. Long Noncoding RNA CPR (Cardiomyocyte Proliferation Regulator) Regulates Cardiomyocyte Proliferation and Cardiac Repair. Circulation. 2019;139:2668–84. - PubMed
-
- Feng J, Li Y, Nie Y. Non-Cardiomyocytes in Heart Regeneration. Curr Drug Targets. 2018;19:1077–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

