Targeting phosphatidylserine for Cancer therapy: prospects and challenges
- PMID: 32802188
- PMCID: PMC7415799
- DOI: 10.7150/thno.45125
Targeting phosphatidylserine for Cancer therapy: prospects and challenges
Abstract
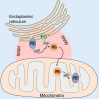
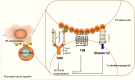
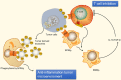
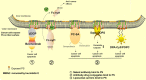
Cancer is a leading cause of mortality and morbidity worldwide. Despite major improvements in current therapeutic methods, ideal therapeutic strategies for improved tumor elimination are still lacking. Recently, immunotherapy has attracted much attention, and many immune-active agents have been approved for clinical use alone or in combination with other cancer drugs. However, some patients have a poor response to these agents. New agents and strategies are needed to overcome such deficiencies. Phosphatidylserine (PS) is an essential component of bilayer cell membranes and is normally present in the inner leaflet. In the physiological state, PS exposure on the external leaflet not only acts as an engulfment signal for phagocytosis in apoptotic cells but also participates in blood coagulation, myoblast fusion and immune regulation in nonapoptotic cells. In the tumor microenvironment, PS exposure is significantly increased on the surface of tumor cells or tumor cell-derived microvesicles, which have innate immunosuppressive properties and facilitate tumor growth and metastasis. To date, agents targeting PS have been developed, some of which are under investigation in clinical trials as combination drugs for various cancers. However, controversial results are emerging in laboratory research as well as in clinical trials, and the efficiency of PS-targeting agents remains uncertain. In this review, we summarize recent progress in our understanding of the physiological and pathological roles of PS, with a focus on immune suppressive features. In addition, we discuss current drug developments that are based on PS-targeting strategies in both experimental and clinical studies. We hope to provide a future research direction for the development of new agents for cancer therapy.
Keywords: T lymphocytes; bavituximab; cancer; immunotherapy; phosphatidylserine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Takeyama H, Beppu T, Higashi T, Kaida T, Arima K, Taki K. et al. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surg Today. 2018;48(4):431–8. - PubMed
-
- Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C. et al. Radiation therapy for the whole breast: Executive summary of an american society for radiation oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–52. - PubMed
-
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. - PubMed
-
- Liu Y, Ding W, Ge H, Ponnusamy M, Wang Q, Hao X. et al. Foxk transcription factors: Regulation and critical role in cancer. Cancer Lett. 2019;458:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

