Kinetic Resolution of Racemic Primary Amines Using Geobacillus stearothermophilus Amine Dehydrogenase Variant
- PMID: 32802214
- PMCID: PMC7422701
- DOI: 10.1002/cctc.201902085
Kinetic Resolution of Racemic Primary Amines Using Geobacillus stearothermophilus Amine Dehydrogenase Variant
Abstract
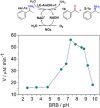
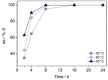
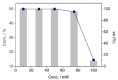
A NADH-dependent engineered amine dehydrogenase from Geobacillus stearothermophilus (LE-AmDH-v1) was applied together with a NADH-oxidase from Streptococcus mutans (NOx) for the kinetic resolution of pharmaceutically relevant racemic α-chiral primary amines. The reaction conditions (e. g., pH, temperature, type of buffer) were optimised to yield S-configured amines with up to >99 % ee.
Keywords: amine dehydrogenases; biocatalysis; kinetic resolution; oxidative deamination; α-chiral amines.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- None
-
- Wittcoff H. A., Rueben B. G., Plotkin J. S., Industrial Organic Chemicals, 2nd ed., Wiley-Interscience, New York, 2004;
-
- Ghislieri D., Turner N. J., Top. Catal. 2013, 57, 284–300;
-
- Constable D. J. C., Dunn P. J., Hayler J. D., Humphrey G. R., Leazer J. J. L., Linderman R. J., Lorenz K., Manley J., Pearlman B. A., Wells A., Zaks A., Zhang T. Y., Green Chem. 2007, 9, 411–420;
-
- Nugent T. C., Chiral amine synthesis: Methods, Developments and Applications, Wiley-VCH, 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
