Efficacy of Fixed-combination Calcipotriene 0.005% and Betamethasone Dipropionate 0.064% Foam for Scalp Plaque Psoriasis: Additional Analysis of a Phase II, Randomized Clinical Study
- PMID: 32802249
- PMCID: PMC7380692
Efficacy of Fixed-combination Calcipotriene 0.005% and Betamethasone Dipropionate 0.064% Foam for Scalp Plaque Psoriasis: Additional Analysis of a Phase II, Randomized Clinical Study
Abstract
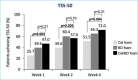
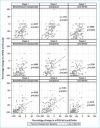
BACKGROUND: There are a variety of treatment options currently available for plaque psoriasis affecting the scalp, yet scalp psoriasis remains one of the most frustrating and difficult-to-manage forms of the disease. OBJECTIVE: We investigated the efficacy of fixed-combination calcipotriene 0.005% plus betamethasone dipropionate 0.064% (Cal/BD) foam for the treatment of scalp psoriasis. METHODS: Additional (including post-hoc) analysis was conducted on data from a Phase II, randomized, double-blind, multicenter study of Cal/BD foam for the treatment of psoriasis vulgaris (NCT01536938). A total of 302 patients, ages 18 years or older, with psoriasis vulgaris of at least mild severity (scalp involvement of at least 10%) were included; 100, 101, and 101 patients were randomized to once-daily Cal/BD foam, Cal foam, or BD foam, respectively. Assessments included a severity score for lesion redness, scaliness, and plaque thickness, modified Psoriasis Area and Severity Index (mPASI) score, proportion of patients with reduction of 50 percent or greater in total sign score (TSS-50), and proportion of patients with at least a 75-percent reduction in mPASI score (mPASI-75). RESULTS: Patients achieved greater improvements in their scalp psoriasis with Cal/BD foam versus BD or Cal foam alone at Week 4 considering mPASI, mPASI-75, and TSS-50 outcomes. After four weeks of treatment, more patients receiving Cal/BD foam had a severity score for redness, scaliness, and thickness indicating "none" or "mild" versus BD foam or Cal foam alone. Improvements on the scalp appear to be consistent with those on the trunk and limbs. CONCLUSION: Scalp lesion severity improved considerably and rapidly with a four-week regimen of Cal/BD foam, suggesting that Cal/BD foam is an effective topical treatment option for scalp psoriasis.
Keywords: Calcipotriene; betamethasone dipropionate; foam; scalp psoriasis; target lesion.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:p-value communications provided medical writing, editing, and publication assistance and was funded by LEO Pharma. DISCLOSURES:Drs. Patel, Veverka, and Hansen are employees of LEO Pharma. Dr. Yamauchi serves as a consultant, speaker, advisory board participant, or investigator for AbbVie, Amgen, Celgene, Dermira, Galderma, Janssen-Ortho, LEO Pharma, Lilly, Medimmune, Menlo, Novartis, Ortho, Pfizer Inc., Regeneron, Sandoz, Sanofi, and Sun. Dr. Alonso-Llamazares serves as a speaker for Celgene, Dermira, Ortho Dermatologics, Eli Lilly and Company, and UCB. Dr. Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development, LLC, LEO Pharma, Ortho Dermatologics, Pfizer Inc., and UCB. and is a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., BMD skincare, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer Inc., Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica.
Figures

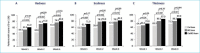


References
-
- van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol. 2001;2(3):159–165. - PubMed
-
- Farber EM, Nall L. Natural history and treatment of scalp psoriasis. Cutis. 1992;49(6):396–400. - PubMed
-
- Kaur I, Handa S, Kumar B. Natural history of psoriasis: a study from the Indian subcontinent. J Dermatol. 1997;24(4):230–234. - PubMed
-
- van de Kerkhof PC, de Hoop D, de Korte J et al. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology. 1998;197(4):326–334. - PubMed
-
- Kragballe K, Menter A, Lebwohl M et al. Long-term management of scalp psoriasis: perspectives from the International Psoriasis Council. J Dermatolog Treat. 2013;24(3):188–192. - PubMed
LinkOut - more resources
Full Text Sources
