Novel zwitterionic vectors: Multi-functional delivery systems for therapeutic genes and drugs
- PMID: 32802271
- PMCID: PMC7403891
- DOI: 10.1016/j.csbj.2020.07.015
Novel zwitterionic vectors: Multi-functional delivery systems for therapeutic genes and drugs
Abstract
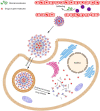
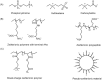
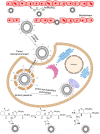
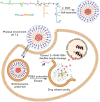
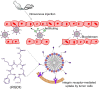
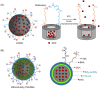
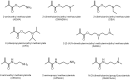
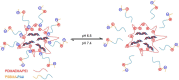
Zwitterions consist of equal molar cationic and anionic moieties and thus exhibit overall electroneutrality. Zwitterionic materials include phosphorylcholine, sulfobetaine, carboxybetaine, zwitterionic amino acids/peptides, and other mix-charged zwitterions that could form dense and stable hydration shells through the strong ion-dipole interaction among water molecules and zwitterions. As a result of their remarkable hydration capability and low interfacial energy, zwitterionic materials have become ideal choices for designing therapeutic vectors to prevent undesired biosorption especially nonspecific biomacromolecules during circulation, which was termed antifouling capability. And along with their great biocompatibility, low cytotoxicity, negligible immunogenicity, systematic stability and long circulation time, zwitterionic materials have been widely utilized for the delivery of drugs and therapeutic genes. In this review, we first summarized the possible antifouling mechanism of zwitterions briefly, and separately introduced the features and advantages of each type of zwitterionic materials. Then we highlighted their applications in stimuli-responsive "intelligent" drug delivery systems as well as tumor-targeting carriers and stressed the multifunctional role they played in therapeutic gene delivery.
Keywords: Antifouling; Biomaterials; Drug delivery; Gene delivery; Zwitterion.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Frokjaer S., Otzen D.E. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. - PubMed
-
- Otsuka H., Nagasaki Y., Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–419. - PubMed
-
- Shuai X., Ai H., Nasongkla N., Kim S., Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415–426. - PubMed
-
- Zhang Z., Xiong X., Wan J., Xiao L., Gan L. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials. 2012;33:7233–7240. - PubMed
-
- Gaberc-Porekar V., Zore I., Podobnik B., Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11:242–250. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
