Water-Soluble Anthraquinone Photocatalysts Enable Methanol-Driven Enzymatic Halogenation and Hydroxylation Reactions
- PMID: 32802571
- PMCID: PMC7418218
- DOI: 10.1021/acscatal.0c01958
Water-Soluble Anthraquinone Photocatalysts Enable Methanol-Driven Enzymatic Halogenation and Hydroxylation Reactions
Abstract
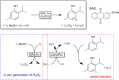
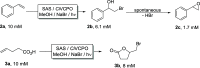
Peroxyzymes simply use H2O2 as a cosubstrate to oxidize a broad range of inert C-H bonds. The lability of many peroxyzymes against H2O2 can be addressed by a controlled supply of H2O2, ideally in situ. Here, we report a simple, robust, and water-soluble anthraquinone sulfonate (SAS) as a promising organophotocatalyst to drive both haloperoxidase-catalyzed halogenation and peroxygenase-catalyzed oxyfunctionalization reactions. Simple alcohols, methanol in particular, can be used both as a cosolvent and an electron donor for H2O2 generation. Very promising turnover numbers for the biocatalysts of up to 318 000 have been achieved.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Burek B. O. O.; Bormann S.; Hollmann F.; Bloh J.; Holtmann D. Hydrogen Peroxide Driven Biocatalysis. Green Chem. 2019, 21, 3232–3249. 10.1039/C9GC00633H. - DOI
-
- Renirie R.; Pierlot C.; Wever R.; Aubry J. M. Singlet Oxygenation in Microemulsion Catalysed by Vanadium Chloroperoxidase. J. Mol. Catal. B: Enzym. 2009, 56, 259–264. 10.1016/j.molcatb.2008.05.014. - DOI
-
- Renirie R.; Pierlot C.; Aubry J.-M.; Hartog A. F.; Schoemaker H. E.; Alsters P. L.; Wever R. Vanadium Chloroperoxidase as a Catalyst for Hydrogen Peroxide Disproportionation to Singlet Oxygen in Mildly Acidic Aqueous Environment. Adv. Synth. Catal. 2003, 345, 849–858. 10.1002/adsc.200303008. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
