Targeting Prostate Cancer Using Intratumoral Cytotopically Modified Interleukin-15 Immunotherapy in a Syngeneic Murine Model
- PMID: 32802803
- PMCID: PMC7394845
- DOI: 10.2147/ITT.S257443
Targeting Prostate Cancer Using Intratumoral Cytotopically Modified Interleukin-15 Immunotherapy in a Syngeneic Murine Model
Abstract
Background: The prostate cancer microenvironment is highly immunosuppressive; immune cells stimulated in the periphery by systemic immunotherapies will be rendered inactive once entering this environment. Immunotherapies for prostate cancer need to break this immune tolerance. We have previously identified interleukin-15 (IL-15) as the only cytokine tested that activates and expands immune cells in the presence of prostate cancer cells. In the current study, we aimed to identify a method of boosting the efficacy of IL-15 in prostate cancer.
Methods: We engineered, by conjugation to a myristoylated peptide, a membrane-localising form of IL-15 (cyto-IL-15) and the checkpoint inhibitor antibodies cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death ligand 1 (PD-L1) (cyto-abs) to enable them to bind to cell surfaces by non-specific anchoring to the phospholipid bilayer. The efficacy of these agents was investigated by intratumoral administration either alone (cyto-IL-15 or cyto-abs) or in combination (cyto-combo) in subcutaneous TRAMP-C2 prostate tumors in C57BL/6J mice and compared with their non-modified equivalents in vivo. Following the survival endpoint, histological analyses and RNA sequencing were performed on the tumors.
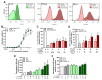
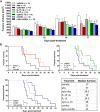
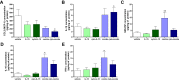
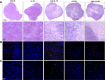
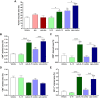
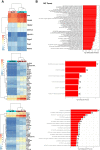
Results: Intratumoral injection of cyto-IL-15 or cyto-combo delayed tumor growth by 50% and increased median survival to 28 and 25 days, respectively, compared with vehicle (17 days), whereas non-modified IL-15 or antibodies alone had no significant effects on tumor growth or survival. Histological analysis showed that cyto-IL-15 and cyto-combo increased necrosis and infiltration of natural killer (NK) cells and CD8 T cells in the tumors compared with vehicle and non-modified agents. Overall, the efficacy of cyto-combo was not superior to that of cyto-IL-15 alone.
Conclusion: We have demonstrated that intratumoral injection of cyto-IL-15 leads to prostate cancer growth delay, induces tumor necrosis and increases survival. Hence, cytotopic modification in combination with intratumoral injection appears to be a promising novel approach for prostate cancer immunotherapy.
Keywords: IL-15; NK cells; checkpoint blockade; cytotopic modification; prostate cancer.
© 2020 Papaevangelou et al.
Conflict of interest statement
Efthymia Papaevangelou, Dorota Smolarek, Richard A Smith, Prokar Dasgupta, and Christine Galustian report a patent, GB 1,913,804.9, pending to King's College London and the Prostate Cancer Research Centre Charity. The authors report no other possible conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

