Effectiveness and Safety of Botulinum Toxin Type A in the Treatment of Androgenetic Alopecia
- PMID: 32802833
- PMCID: PMC7424364
- DOI: 10.1155/2020/1501893
Effectiveness and Safety of Botulinum Toxin Type A in the Treatment of Androgenetic Alopecia
Abstract
Background: Androgenetic alopecia (AGA) represents the most frequent clinical complaint encountered by dermatologists and is characterized by a progressive miniaturization of the hair follicle. However, the efficacy and safety of current medical treatment remain limited, and more personalized therapeutic approaches for AGA are needed. Therefore, the present study is aimed at investigating the efficacy and safety of botulinum toxin type A (BTA) in patients with AGA.
Methods: 63 patients with AGA meeting the inclusion criteria were included in this study and treated with BTA injection or BTA injection combined with oral finasteride (FNS). In the scalp, 30 sites were injected with 100 U of BTA in each site and patients received BTA after every 3 months for a total of 4 times. Hair counts, head photographs, evaluation scores, and self-assessment were assessed in patients with AGA.
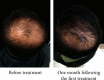
Results: Hair counts in both groups at all time points were significantly higher as compared with those before treatment. After 4 times of treatment, hair counts in the BTA+FNS group were higher than those in the BTA group. Hair growth and density were significantly augmented, and the area of hair loss was attenuated after each treatment as revealed by head photographs. The effective rates of BTA and BTA+FNS groups were 73.3% and 84.8%, respectively, following 4 times treatment.
Conclusion: BTA is a safe and effective therapeutic strategy for the treatment of AGA without adverse effects, and BTA combined with FNS exhibited a superior therapeutic effect than BTA alone.
Copyright © 2020 Yaguang Zhou et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures




References
-
- Norwood O. T. Incidence of female androgenetic alopecia (female pattern alopecia) Dermatologic Surgery. 2001;27(1):53–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

