Blood Monocyte Phenotype Fingerprint of Stable Coronary Artery Disease: A Cross-Sectional Substudy of SMARTool Clinical Trial
- PMID: 32802883
- PMCID: PMC7403909
- DOI: 10.1155/2020/8748934
Blood Monocyte Phenotype Fingerprint of Stable Coronary Artery Disease: A Cross-Sectional Substudy of SMARTool Clinical Trial
Abstract
Background and aims: Atherosclerosis is an inflammatory disease with long-lasting activation of innate immunity and monocytes are the main blood cellular effectors. We aimed to investigate monocyte phenotype (subset fraction and marker expression) at different stages of coronary atherosclerosis in stable coronary artery disease (CAD) patients.
Methods: 73 patients with chronic coronary syndrome were evaluated by CT coronary angiography (CTCA) and classified by maximal diameter stenosis of major vessels into three groups of CAD severity: CAD1 (no CAD/minimal CAD, n° = 30), CAD2 (non-obstructive CAD, n° = 21), and CAD3 (obstructive CAD, n° = 22). Flow cytometry for CD14, CD16, and CCR2 was used to quantify Mon1, Mon2, and Mon3 subsets. Expression of CD14, CD16, CD18, CD11b, HLA-DR, CD163, CCR2, CCR5, CX3CR1, and CXCR4 was also measured. Adhesion molecules and cytokines were quantified by ELISA.
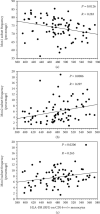
Results: Total cell count and fraction of Mon2 were higher in CAD2 and CAD3 compared to CAD1. By multivariate regression analysis, Mon2 cell fraction and Mon2 expression of CX3CR1, CD18, and CD16 showed a statistically significant and independent increase, parallel to stenosis severity, from CAD1 to CAD2 and CAD3 groups. A similar trend was also present for CX3CR1 and HLA-DR expressions on total monocyte population. A less calcified plaque composition was associated to a higher Mon2 expression of CD16 and higher TNF-α levels. IL-10 levels were lower at greater stenosis severity, while the IFN-γ/IL-10 ratio, a marker of a systemic pro-inflammatory imbalance, was directly correlated to stenosis degree and number of noncalcified plaques.
Conclusions: The results of this study suggest that a specific pattern of inflammation-correlated monocyte marker expression is associated to higher stenosis severity and less calcified lesions in stable CAD. The clinical trial Identifier is NCT04448691.
Copyright © 2020 Silverio Sbrana et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

