Purification of Human CD34+CD90+ HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy
- PMID: 32802914
- PMCID: PMC7424231
- DOI: 10.1016/j.omtm.2020.07.010
Purification of Human CD34+CD90+ HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy
Abstract
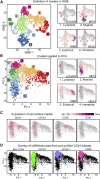
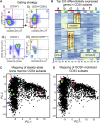
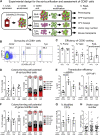
Hematopoietic stem cell (HSC) gene therapy has the potential to cure many genetic, malignant, and infectious diseases. We have shown in a nonhuman primate gene therapy and transplantation model that the CD34+CD90+ cell fraction was exclusively responsible for multilineage engraftment and hematopoietic reconstitution. In this study, we show the translational potential of this HSC-enriched CD34 subset for lentivirus-mediated gene therapy. Alternative HSC enrichment strategies include the purification of CD133+ cells or CD38low/- subsets of CD34+ cells from human blood products. We directly compared these strategies to the isolation of CD90+ cells using a good manufacturing practice (GMP) grade flow-sorting protocol with clinical applicability. We show that CD90+ cell selection results in about 30-fold fewer target cells in comparison to CD133+ or CD38low/- CD34+ hematopoietic stem and progenitor cell (HSPC) subsets without compromising the engraftment potential in vivo. Single-cell RNA sequencing confirmed nearly complete depletion of lineage-committed progenitor cells in CD90+ fractions compared to alternative selections. Importantly, lentiviral transduction efficiency in purified CD90+ cells resulted in up to 3-fold higher levels of engrafted gene-modified blood cells. These studies should have important implications for the manufacturing of patient-specific HSC gene therapy and gene-engineered cell products.
Keywords: CD90 CD34; Closed System Application; Gene Therapy; Hematopoietic Stem Cells; Lentivirus Transduction; Mouse xenograft transplants; Stem Cell Enrichment / Cell Sorting; single cell RNA sequencing.
© 2020.
Figures



Similar articles
-
Isolation of a Highly Purified HSC-enriched CD34+CD90+CD45RA- Cell Subset for Allogeneic Transplantation in the Nonhuman Primate Large-animal Model.Transplant Direct. 2020 Jul 15;6(8):e579. doi: 10.1097/TXD.0000000000001029. eCollection 2020 Aug. Transplant Direct. 2020. PMID: 33134503 Free PMC article.
-
Preferential Expansion of Human CD34+CD133+CD90+ Hematopoietic Stem Cells Enhances Gene-Modified Cell Frequency for Gene Therapy.Hum Gene Ther. 2022 Feb;33(3-4):188-201. doi: 10.1089/hum.2021.089. Epub 2022 Jan 7. Hum Gene Ther. 2022. PMID: 34486377
-
CD90-targeted lentiviral vectors for HSC gene therapy.Mol Ther. 2023 Oct 4;31(10):2901-2913. doi: 10.1016/j.ymthe.2023.08.003. Epub 2023 Aug 7. Mol Ther. 2023. PMID: 37550965 Free PMC article.
-
CD34+ CD90+ cells and late hematopoietic reconstitution after autologous peripheral blood stem cell transplantation.Leuk Lymphoma. 2004 Apr;45(4):661-8. doi: 10.1080/1042819031000140997. Leuk Lymphoma. 2004. PMID: 15160937 Review.
-
Gene transfer into nonhuman primate hematopoietic stem cells: implications for gene therapy.Stem Cells. 2001;19(1):12-23. doi: 10.1634/stemcells.19-1-12. Stem Cells. 2001. PMID: 11209087 Review.
Cited by
-
Exploring the potential of predicted miRNAs on the genes involved in the expansion of hematopoietic stem cells.Sci Rep. 2024 Jul 5;14(1):15551. doi: 10.1038/s41598-024-66614-9. Sci Rep. 2024. PMID: 38969714 Free PMC article.
-
Therapy Development by Genome Editing of Hematopoietic Stem Cells.Cells. 2021 Jun 14;10(6):1492. doi: 10.3390/cells10061492. Cells. 2021. PMID: 34198536 Free PMC article. Review.
-
Targeted, safe, and efficient gene delivery to human hematopoietic stem and progenitor cells in vivo using the engineered AVID adenovirus vector platform.Mol Ther. 2024 Jan 3;32(1):103-123. doi: 10.1016/j.ymthe.2023.10.023. Epub 2023 Nov 2. Mol Ther. 2024. PMID: 37919899 Free PMC article.
-
cMPL-based purification and depletion of human hematopoietic stem cells: implications for pretransplant conditioning.Blood. 2025 Jun 19;145(25):2978-2991. doi: 10.1182/blood.2024024636. Blood. 2025. PMID: 40009502
-
Choosing the right mouse model: comparison of humanized NSG and NBSGW mice for in vivo HSC gene therapy.Blood Adv. 2024 Feb 27;8(4):916-926. doi: 10.1182/bloodadvances.2023011371. Blood Adv. 2024. PMID: 38113461 Free PMC article.
References
-
- Notta F., Doulatov S., Laurenti E., Poeppl A., Jurisica I., Dick J.E. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. - PubMed
-
- Doulatov S., Notta F., Eppert K., Nguyen L.T., Ohashi P.S., Dick J.E. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat. Immunol. 2010;11:585–593. - PubMed
-
- Barnett D., Janossy G., Lubenko A., Matutes E., Newland A., Reilly J.T., General Haematology Task Force of the British Committee for Standards in Haematology Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. Clin. Lab. Haematol. 1999;21:301–308. - PubMed
-
- Trébéden-Negre H., Rosenzwajg M., Tanguy M.L., Lefrere F., Azar N., Heshmati F., Belhocine R., Vernant J.P., Klatzmann D., Norol F. Delayed recovery after autologous peripheral hematopoietic cell transplantation: potential effect of a high number of total nucleated cells in the graft. Transfusion. 2010;50:2649–2659. - PubMed
-
- Peterson C.W., Haworth K.G., Burke B.P., Polacino P., Norman K.K., Adair J.E., Hu S.L., Bartlett J.S., Symonds G.P., Kiem H.P. Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS. Mol. Ther. Methods Clin. Dev. 2016;3:16007. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

