Adapting techniques for calcium imaging in muscles of adult Brugia malayi
- PMID: 32803437
- PMCID: PMC7891862
- DOI: 10.1007/s10158-020-00247-1
Adapting techniques for calcium imaging in muscles of adult Brugia malayi
Abstract
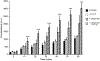
Brugia malayi is a human filarial nematode parasite that causes lymphatic filariasis or 'elephantiasis' a disfiguring neglected tropical disease. This parasite is a more tractable nematode parasite for the experimental study of anthelmintic drugs and has been studied with patch-clamp and RNAi techniques. Unlike in C. elegans however, calcium signaling in B. malayi or other nematode parasites has not been achieved, limiting the studies of the mode of action of anthelmintic drugs. We describe here the development of calcium imaging methods that allow us to characterize changes in cellular calcium in the muscles of B. malayi. This is a powerful technique that can help in elucidating the mode of action of selected anthelmintics. We developed two approaches that allow the recording of calcium signals in the muscles of adult B. malayi: (a) soaking the muscles with Fluo-3AM, promoting large-scale imaging of multiple cells simultaneously and, (b) direct insertion of Fluo-3 using microinjection, providing the possibility of performing dual calcium and electrophysiological recordings. Here, we describe the techniques used to optimize dye entry into the muscle cells and demonstrate that detectable increases in Fluo-3 fluorescence to elevated calcium concentrations can be achieved in B. malayi using both techniques.
Keywords: B. malayi; Calcium imaging; Fluo-3; Muscle.
Conflict of interest statement
Figures


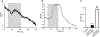

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

