A lab-on-a-chip model of glaucoma
- PMID: 32803874
- PMCID: PMC7559618
- DOI: 10.1002/brb3.1799
A lab-on-a-chip model of glaucoma
Abstract
Aims: We developed a glaucoma-on-a-chip model to evaluate the viability of retinal ganglion cells (RGCs) against high pressure and the potential effect of neuroprotection.
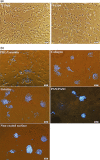
Methods: A three-layered chip consisting of interconnecting microchannels and culture wells was designed and fabricated from poly-methyl methacrylate sheets. The bottom surface of the wells was modified by air plasma and coated with different membranes to provide a suitable extracellular microenvironment. RGCs were purified from postnatal Wistar rats by magnetic assisted cell sorting up to 70% and characterized by flow cytometry and immunocytochemistry. The cultured RGCs were exposed to normal (15 mmHg) or elevated pressure (33 mmHg) for 6, 12, 24, 36, and 48 hr, with and without adding brain-derived neurotrophic factor (BDNF) or a novel BDNF mimetic (RNYK).
Results: Multiple inlet ports allow culture media and gas into the wells under elevated hydrostatic pressure. PDL/laminin formed the best supporting membrane. RGC survival rates were 85%, 78%, 70%, 67%, and 61% under normal pressure versus 40%, 22%, 18%, 12%, and 10% under high pressure at 6, 12, 24, 36, and 48 hr, respectively. BDNF and RNYK separately reduced RGC death rates about twofold under both normal and elevated pressures.
Conclusion: This model recapitulated the effects of elevated pressure over relatively short time periods and demonstrated the neuroprotective effects of BDNF and RNYK.
Keywords: glaucoma; hydrostatic pressure; lab-on-a-chip; microenvironment; retinal ganglion cell.
© 2020 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures






References
-
- Bamshad, A. , Nikfarjam, A. , & Khaleghi, H. (2016). A new simple and fast thermally‐solvent assisted method to bond PMMA–PMMA in micro‐fluidics devices. Journal of Micromechanics and Microengineering, 26(6), 1–12. 10.1088/0960-1317/26/6/065017 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

