Ferroptosis: machinery and regulation
- PMID: 32804006
- PMCID: PMC8496712
- DOI: 10.1080/15548627.2020.1810918
Ferroptosis: machinery and regulation
Abstract
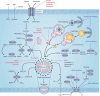
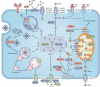
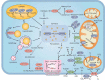
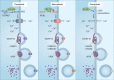
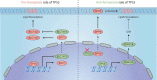
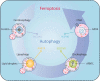
Ferroptosis is an iron-dependent, non-apoptotic form of regulated cell death caused by lipid peroxidation, which is controlled by integrated oxidation and antioxidant systems. The iron-containing enzyme lipoxygenase is the main promoter of ferroptosis by producing lipid hydroperoxides, and its function relies on the activation of ACSL4-dependent lipid biosynthesis. In contrast, the selenium-containing enzyme GPX4 is currently recognized as a central repressor of ferroptosis, and its activity depends on glutathione produced from the activation of the cystine-glutamate antiporter SLC7A11. Many metabolic (especially involving iron, lipids, and amino acids) and degradation pathways (macroautophagy/autophagy and the ubiquitin-proteasome system) orchestrate the complex ferroptotic response through direct or indirect regulation of iron accumulation or lipid peroxidation. Although the detailed mechanism of membrane injury during ferroptosis remains a mystery, ESCRT III-mediated plasma membrane repair can make cells resistant to ferroptosis. Here, we review the recent rapid progress in understanding the molecular mechanisms of ferroptosis and focus on the epigenetic, transcriptional, and posttranslational regulation of this process.Abbreviations: 2ME: beta-mercaptoethanol; α-KG: α-ketoglutarate; ccRCC: clear cell renal cell carcinoma; EMT: epithelial-mesenchymal transition; FAO: fatty acid beta-oxidation; GSH: glutathione; MEFs: mouse embryonic fibroblasts; MUFAs: monounsaturated fatty acids; NO: nitric oxide; NOX: NADPH oxidase; PPP: pentose phosphate pathway; PUFA: polyunsaturated fatty acid; RCD: regulated cell death; RNS: reactive nitrogen species; ROS: reactive oxygen species; RTAs: radical-trapping antioxidants; UPS: ubiquitin-proteasome system; UTR: untranslated region.
Keywords: Autophagy; Ferroptosis; cell death.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Weinlich R, Oberst A, Beere HM, et al. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18(2):127–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
