A novel biparatopic hybrid antibody-ACE2 fusion that blocks SARS-CoV-2 infection: implications for therapy
- PMID: 32804015
- PMCID: PMC7531490
- DOI: 10.1080/19420862.2020.1804241
A novel biparatopic hybrid antibody-ACE2 fusion that blocks SARS-CoV-2 infection: implications for therapy
Abstract
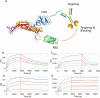
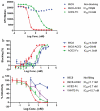
In the absence of a proven effective vaccine preventing infection by SARS-CoV-2, or a proven drug to treat COVID-19, the positive results of passive immune therapy using convalescent serum provide a strong lead. We have developed a new class of tetravalent, biparatopic therapy, 89C8-ACE2. It combines the specificity of a monoclonal antibody (89C8) that recognizes the relatively conserved N-terminal domain of the viral Spike (S) glycoprotein, and the ectodomain of ACE2, which binds to the receptor-binding domain of S. This molecule shows exceptional performance in vitro, inhibiting the interaction of recombinant S1 to ACE2 and transduction of ACE2-overexpressing cells by S-pseudotyped lentivirus with IC50s substantially below 100 pM, and with potency approximately 100-fold greater than ACE2-Fc itself. Moreover, 89C8-ACE2 was able to neutralize authentic viral infection in a standard 96-h co-incubation assay at low nanomolar concentrations, making this class of molecule a promising lead for therapeutic applications.
Keywords: Biparatopic; COVID-19; SARS-CoV-2; antibody-ACE2 fusion; neutralizing antibody.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
