An Open-Label, 8-Week Study of Safety and Efficacy of Pimavanserin Treatment in Adults with Parkinson's Disease and Depression
- PMID: 32804101
- PMCID: PMC7683094
- DOI: 10.3233/JPD-202058
An Open-Label, 8-Week Study of Safety and Efficacy of Pimavanserin Treatment in Adults with Parkinson's Disease and Depression
Abstract
Background: Many patients with Parkinson's disease (PD) experience depression.
Objective: Evaluate pimavanserin treatment for depression in patients with PD.
Methods: Pimavanserin was administered as monotherapy or adjunctive therapy to a selective serotonin reuptake inhibitor or serotonin/noradrenaline reuptake inhibitor in this 8-week, single-arm, open-label phase 2 study (NCT03482882). The primary endpoint was change from baseline to week 8 in Hamilton Depression Scale-17-item version (HAMD-17) score. Safety, including collection of adverse events and the Mini-Mental State Examination (MMSE) and Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS III) scores, was assessed in patients who received ≥1 pimavanserin dose.
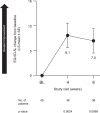
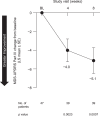
Results: Efficacy was evaluated in 45 patients (21 monotherapy, 24 adjunctive therapy). Mean (SE) baseline HAMD-17 was 19.2 (3.1). Change from baseline to week 8 (least squares [LS] mean [SE]) in the HAMD-17 was -10.8 (0.63) (95% CI, -12.0 to -9.5; p < 0.0001) with significant improvement seen at week 2 (p < 0.0001) and for both monotherapy (week 8, -11.2 [0.99]) and adjunctive therapy (week 8,-10.2 [0.78]). Most patients (60.0%) had ≥50% improvement at week 8, and 44.4% of patients reached remission (HAMD-17 score ≤7). Twenty-one of 47 patients experienced 42 treatment-emergent adverse events; the most common by system organ class were gastrointestinal (n = 7; 14.9%) and psychiatric (n = 7; 14.9%). No negative effects were observed on MMSE or MDS-UPDRS Part III.
Conclusion: In this 8-week, single-arm, open-label study, pimavanserin as monotherapy or adjunctive therapy was well tolerated and associated with early and sustained improvement of depressive symptoms in patients with PD.
Keywords: Parkinson’s disease; adjunctive therapy; dementia; depression; monotherapy; pimavanserin.
Conflict of interest statement
DD, BC, LJ, RN, VA: are employees of and hold stock and/or stock options in ACADIA Pharmaceuticals Inc.
JCN: was an employee of ACADIA Pharmaceuticals Inc. at the time of this study.
GA: has received research support from Accera, Allergan, Axovant, Eisai, Genentech, Intra- Cellular, Janssen, Lundbeck, Neurim, Neurotrope, Novartis, Otsuka, Roche, Suven, and TransTech and has served on the speakers bureau or as a consultant for ACADIA, Alkermes, Allergan, Avanir, Janssen, Lundbeck, Merck, Nestlé, Otsuka, Sunovion, Takeda, and Vanda.
JLA: has received research support from Abbott, AbbVie, ACADIA, Biogen, Boston Scientific, Denali, Impax, NeuroDerm, Sunovion, and Theravance and has received honoraria from Abbott, AbbVie, Acorda, Adamas, Allergan, Boston Scientific, Medtronic, Teva, and US WorldMeds.
MC is a consultant to ACADIA, Kyowa Kirin, Sunovion, and Reviva.
Figures





References
-
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP (2001) Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 13, 187–196. - PubMed
-
- Rocha FL, Murad MG, Stumpf BP, Hara C, Fuzikawa CJJop (2013) Antidepressants for depression in Parkinson’s disease: Systematic review and meta-analysis, 27, 417–423. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

