Mitochondrial DNA Manipulations Affect Tau Oligomerization
- PMID: 32804126
- PMCID: PMC7962146
- DOI: 10.3233/JAD-200286
Mitochondrial DNA Manipulations Affect Tau Oligomerization
Abstract
Background: Mitochondrial dysfunction and tau aggregation occur in Alzheimer's disease (AD), and exposing cells or rodents to mitochondrial toxins alters their tau.
Objective: To further explore how mitochondria influence tau, we measured tau oligomer levels in human neuronal SH-SY5Y cells with different mitochondrial DNA (mtDNA) manipulations.
Methods: Specifically, we analyzed cells undergoing ethidium bromide-induced acute mtDNA depletion, ρ0 cells with chronic mtDNA depletion, and cytoplasmic hybrid (cybrid) cell lines containing mtDNA from AD subjects.
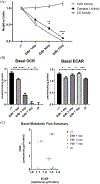
Results: We found cytochrome oxidase activity was particularly sensitive to acute mtDNA depletion, evidence of metabolic re-programming in the ρ0 cells, and a relatively reduced mtDNA content in cybrids generated through AD subject mitochondrial transfer. In each case tau oligomer levels increased, and acutely depleted and AD cybrid cells also showed a monomer to oligomer shift.
Conclusion: We conclude a cell's mtDNA affects tau oligomerization. Overlapping tau changes across three mtDNA-manipulated models establishes the reproducibility of the phenomenon, and its presence in AD cybrids supports its AD-relevance.
Keywords: Alzheimer’s disease; mitochondria; mitochondrial DNA; oligomers; tau.
Conflict of interest statement
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
Figures






References
-
- Swerdlow RH (2007) Is aging part of Alzheimer’s disease, or is Alzheimer’s disease part of aging? Neurobiol Aging 28, 1465–1480. - PubMed
-
- Atlante A, Amadoro G, Bobba A, de Bari L, Corsetti V, et al. (2008) A peptide containing residues 26–44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim Biophys Acta 1777, 1289–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

