In vivo testing of the low-flow CO2 removal application of a compact, platform respiratory device
- PMID: 32804310
- PMCID: PMC7429452
- DOI: 10.1186/s40635-020-00329-9
In vivo testing of the low-flow CO2 removal application of a compact, platform respiratory device
Abstract
Background: Non-invasive and lung-protective ventilation techniques may improve outcomes for patients with an acute exacerbation of chronic obstructive pulmonary disease or moderate acute respiratory distress syndrome by reducing airway pressures. These less invasive techniques can fail due to hypercapnia and require transitioning patients to invasive mechanical ventilation. Extracorporeal CO2 removal devices remove CO2 independent of the lungs thereby controlling the hypercapnia and permitting non-invasive or lung-protective ventilation techniques. We are developing the Modular Extracorporeal Lung Assist System as a platform technology capable of providing three levels of respiratory assist: adult and pediatric full respiratory support and adult low-flow CO2 removal. The objective of this study was to evaluate the in vivo performance of our device to achieve low-flow CO2 removal.
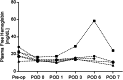
Methods: The Modular Extracorporeal Lung Assist System was connected to 6 healthy sheep via a 15.5 Fr dual-lumen catheter placed in the external jugular vein. The animals were recovered and tethered within a pen while supported by the device for 7 days. The pump speed was set to achieve a targeted blood flow of 500 mL/min. The extracorporeal CO2 removal rate was measured daily at a sweep gas independent regime. Hematological parameters were measured pre-operatively and regularly throughout the study. Histopathological samples of the end organs were taken at the end of each study.
Results: All animals survived the surgery and generally tolerated the device well. One animal required early termination due to a pulmonary embolism. Intra-device thrombus formation occurred in a single animal due to improper anticoagulation. The average CO2 removal rate (normalized to an inlet pCO2 of 45 mmHg) was 75.6 ± 4.7 mL/min and did not significantly change over the course of the study (p > 0.05). No signs of consistent hemolysis or end organ damage were observed.
Conclusion: These in vivo results indicate positive performance of the Modular Extracorporeal Lung Assist System as a low-flow CO2 removal device.
Keywords: Carbon dioxide; Extracorporeal CO2 removal; Hypercapnia.
Conflict of interest statement
W.J.F. chairs the Scientific Advisory Board and is a founder of ALung Technologies, in which he has an equity interest. No other authors have conflicts of interest to report.
Figures



Similar articles
-
Extracorporeal lung support technologies - bridge to recovery and bridge to lung transplantation in adult patients: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(5):1-47. Epub 2010 Apr 1. Ont Health Technol Assess Ser. 2010. PMID: 23074408 Free PMC article.
-
In vivo carbon dioxide clearance of a low-flow extracorporeal carbon dioxide removal circuit in patients with acute exacerbations of chronic obstructive pulmonary disease.Perfusion. 2020 Jul;35(5):436-441. doi: 10.1177/0267659119896531. Epub 2020 Jan 11. Perfusion. 2020. PMID: 31928313
-
Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting.Membranes (Basel). 2020 Dec 23;11(1):8. doi: 10.3390/membranes11010008. Membranes (Basel). 2020. PMID: 33374762 Free PMC article.
-
The use of extracorporeal CO2 removal in acute respiratory failure.Ann Intensive Care. 2021 Mar 11;11(1):43. doi: 10.1186/s13613-021-00824-6. Ann Intensive Care. 2021. PMID: 33709318 Free PMC article. Review.
-
Extracorporeal Lung Support for Hypercapnic Ventilatory Failure.Respir Care. 2018 Sep;63(9):1174-1179. doi: 10.4187/respcare.06277. Respir Care. 2018. PMID: 30166412 Review.
Cited by
-
Alkaline Liquid Ventilation of the Membrane Lung for Extracorporeal Carbon Dioxide Removal (ECCO2R): In Vitro Study.Membranes (Basel). 2021 Jun 22;11(7):464. doi: 10.3390/membranes11070464. Membranes (Basel). 2021. PMID: 34206672 Free PMC article.
-
Epoxy silane sulfobetaine block copolymers for simple, aqueous thromboresistant coating on ambulatory assist lung devices.J Biomed Mater Res A. 2024 Jan;112(1):99-109. doi: 10.1002/jbm.a.37619. Epub 2023 Sep 26. J Biomed Mater Res A. 2024. PMID: 37929658 Free PMC article.
References
-
- Slutsky A, Ranieri V (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136. https://doi.org/10.1056/NEJMra1208707 - PubMed
-
- Brochard L, Mancebo J, Wysocki M, et al (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822. https://doi.org/10.1056/NEJM199509283331301 - PubMed
-
- Calverley PMA (2003) Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J 22:26s–30s. https://doi.org/10.1183/09031936.03.00030103 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

