The Essential Medicinal Chemistry of Cannabidiol (CBD)
- PMID: 32804502
- PMCID: PMC7666069
- DOI: 10.1021/acs.jmedchem.0c00724
The Essential Medicinal Chemistry of Cannabidiol (CBD)
Abstract
This Perspective of the published essential medicinal chemistry of cannabidiol (CBD) provides evidence that the popularization of CBD-fortified or CBD-labeled health products and CBD-associated health claims lacks a rigorous scientific foundation. CBD's reputation as a cure-all puts it in the same class as other "natural" panaceas, where valid ethnobotanicals are reduced to single, purportedly active ingredients. Such reductionist approaches oversimplify useful, chemically complex mixtures in an attempt to rationalize the commercial utility of natural compounds and exploit the "natural" label. Literature evidence associates CBD with certain semiubiquitous, broadly screened, primarily plant-based substances of undocumented purity that interfere with bioassays and have a low likelihood of becoming therapeutic agents. Widespread health challenges and pandemic crises such as SARS-CoV-2 create circumstances under which scientists must be particularly vigilant about healing claims that lack solid foundational data. Herein, we offer a critical review of the published medicinal chemistry properties of CBD, as well as precise definitions of CBD-containing substances and products, distilled to reveal the essential factors that impact its development as a therapeutic agent.
Figures
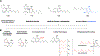
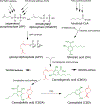
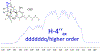
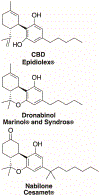
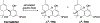
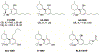

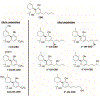
References
-
- Cannabidiol Market Size Analysis | CBD Industry Growth Report, 2025 https://www.grandviewresearch.com/industry-analysis/cannabidiol-cbd-market (accessed Apr 22, 2020).
-
- Dorbian I CBD Market Could Reach $20 Billion By 2024, Says New Study. Forbes Magazine Forbes May 20, 2019.
-
- Yang Y; Zhang Z; Li S; Ye X; Li X; He K Synergy Effects of Herb Extracts: Pharmacokinetics and Pharmacodynamic Basis. Fitoterapia 2014, 92, 133–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

