De novo phosphoinositide synthesis in zebrafish is required for triad formation but not essential for myogenesis
- PMID: 32804943
- PMCID: PMC7430711
- DOI: 10.1371/journal.pone.0231364
De novo phosphoinositide synthesis in zebrafish is required for triad formation but not essential for myogenesis
Abstract
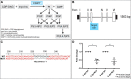
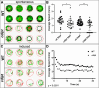
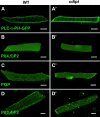
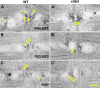
Phosphoinositides (PIPs) and their regulatory enzymes are key players in many cellular processes and are required for aspects of vertebrate development. Dysregulated PIP metabolism has been implicated in several human diseases, including a subset of skeletal myopathies that feature structural defects in the triad. The role of PIPs in skeletal muscle formation, and particularly triad biogenesis, has yet to be determined. CDP-diacylglycerol-inositol 3-phosphatidyltransferase (CDIPT) catalyzes the formation of phosphatidylinositol, which is the base of all PIP species. Loss of CDIPT should, in theory, result in the failure to produce PIPs, and thus provide a strategy for establishing the requirement for PIPs during embryogenesis. In this study, we generated cdipt mutant zebrafish and determined the impact on skeletal myogenesis. Analysis of cdipt mutant muscle revealed no apparent global effect on early muscle development. However, small but significant defects were observed in triad size, with T-tubule area, inter terminal cisternae distance and gap width being smaller in cdipt mutants. This was associated with a decrease in motor performance. Overall, these data suggest that myogenesis in zebrafish does not require de novo PIP synthesis but does implicate a role for CDIPT in triad formation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish.Hepatology. 2011 Aug;54(2):452-62. doi: 10.1002/hep.24349. Epub 2011 May 2. Hepatology. 2011. PMID: 21488074 Free PMC article.
-
Phosphatidylinositol synthase is required for lens structural integrity and photoreceptor cell survival in the zebrafish eye.Exp Eye Res. 2011 Oct;93(4):460-74. doi: 10.1016/j.exer.2011.06.010. Epub 2011 Jun 23. Exp Eye Res. 2011. PMID: 21722635 Free PMC article.
-
Measuring Phosphatidylinositol Generation on Biological Membranes.Methods Mol Biol. 2016;1376:239-46. doi: 10.1007/978-1-4939-3170-5_20. Methods Mol Biol. 2016. PMID: 26552689
-
Phosphoinositide metabolism and Ca2+ oscillation.Biochemistry (Mosc). 1998 Jan;63(1):38-46. Biochemistry (Mosc). 1998. PMID: 9526093 Review.
-
Signaling roles of phosphoinositides in the retina.J Lipid Res. 2021;62:100041. doi: 10.1194/jlr.TR120000806. Epub 2021 Feb 6. J Lipid Res. 2021. PMID: 32540927 Free PMC article. Review.
Cited by
-
X-linked myopathy with excessive autophagy: characterization and therapy testing in a zebrafish model.EMBO Mol Med. 2025 Apr;17(4):823-840. doi: 10.1038/s44321-025-00204-8. Epub 2025 Feb 24. EMBO Mol Med. 2025. PMID: 39994482 Free PMC article.
-
Comprehensive phenotypic characterization of an allelic series of zebrafish models of NEB-related nemaline myopathy.Hum Mol Genet. 2024 Jun 5;33(12):1036-1054. doi: 10.1093/hmg/ddae033. Hum Mol Genet. 2024. PMID: 38493359 Free PMC article.
-
A Hypothesis: Metabolic Contributions to 16p11.2 Deletion Syndrome.Bioessays. 2025 Mar;47(3):e202400177. doi: 10.1002/bies.202400177. Epub 2024 Dec 29. Bioessays. 2025. PMID: 39988938 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

