Fixed vs adjusted-dose benznidazole for adults with chronic Chagas disease without cardiomyopathy: A systematic review and meta-analysis
- PMID: 32804966
- PMCID: PMC7451967
- DOI: 10.1371/journal.pntd.0008529
Fixed vs adjusted-dose benznidazole for adults with chronic Chagas disease without cardiomyopathy: A systematic review and meta-analysis
Abstract
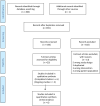
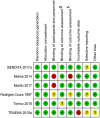
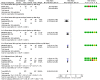
Chagas disease is a neglected disease that remains a public health threat, particularly in Latin America. The most important treatment options are nitroimidazole derivatives, such as nifurtimox and benznidazole (BZN). Some studies suggest that for adults seropositive to T. cruzi but without clinically evident chronic Chagas cardiomyopathy (CCC), a simple fixed-dose scheme of BZN could be equivalent to a weight-adjusted dose. We compared the efficacy and safety of a fixed dose of BZN with an adjusted dose for T. cruzi seropositive adults without CCC. We used the Cochrane methods, and reported according to the PRISMA statement. We included randomized controlled trials (RCTs) allocating participants to fixed and/or adjusted doses of BZN for T. cruzi seropositive adults without CCC. We searched (December 2019) Cochrane, MEDLINE, EMBASE, LILACS, Clinicaltrials.gov, and International Clinical Trials Registry Platform (ICTRP), and contacted Chagas experts. Selection, data extraction, and risk of bias assessment, using the Cochrane tool, were performed independently by pairs of reviewers. Discrepancies were solved by consensus within the team. Primary outcomes were parasite-related outcomes and efficacy or patient-related safety outcomes. We conducted a meta-analysis using RevMan 5.3 software and used GRADE summary of finding tables to present the certainty of evidence by outcome. We identified 655 records through our search strategy and 10 studies (four of them ongoing) met our inclusion criteria. We did not find any study directly comparing fixed vs adjusted doses of BZN, however, some outcomes allowed subgroup comparisons between fixed and adjusted doses of BZN against placebo. Moderate-certainty evidence suggests no important subgroup differences for positive PCR at one year and for three safety outcomes (drug discontinuation, peripheral neuropathy, and mild rash). The same effect was observed for any serious adverse events (low-certainty evidence). All subgroups showed similar effects (I2 0% for all these subgroup comparisons but 32% for peripheral neuropathy), supporting the equivalence of BZN schemes. We conclude that there is no direct evidence comparing fixed and adjusted doses of BZN. Based on low to very low certainty of evidence for critical clinical outcomes and moderate certainty of evidence for important outcomes, fixed and adjusted doses may be equivalent in terms of safety and efficacy. An individual patient data network meta-analysis could better address this issue.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Direct evidence gap on fixed versus adjusted-dose benznidazole for adults with chronic Chagas disease without cardiomyopathy: Systematic review and individual patient data meta-analysis.Trop Med Int Health. 2023 Jan;28(1):2-16. doi: 10.1111/tmi.13831. Epub 2022 Dec 1. Trop Med Int Health. 2023. PMID: 36420767
-
Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection).Cochrane Database Syst Rev. 2020 Dec 11;12(12):CD004102. doi: 10.1002/14651858.CD004102.pub3. Cochrane Database Syst Rev. 2020. PMID: 33305846 Free PMC article.
-
Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial.Trials. 2019 Jul 15;20(1):431. doi: 10.1186/s13063-019-3423-3. Trials. 2019. PMID: 31307503 Free PMC article.
-
Short-course Benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): a non-inferiority randomized controlled trial study protocol.Reprod Health. 2020 Aug 24;17(1):128. doi: 10.1186/s12978-020-00972-1. Reprod Health. 2020. PMID: 32831069 Free PMC article.
-
Recent clinical trials for the etiological treatment of chronic chagas disease: advances, challenges and perspectives.J Eukaryot Microbiol. 2015 Jan-Feb;62(1):149-56. doi: 10.1111/jeu.12184. Epub 2014 Nov 13. J Eukaryot Microbiol. 2015. PMID: 25284065 Review.
Cited by
-
SBC Guideline on the Diagnosis and Treatment of Patients with Cardiomyopathy of Chagas Disease - 2023.Arq Bras Cardiol. 2023 Jun 26;120(6):e20230269. doi: 10.36660/abc.20230269. Arq Bras Cardiol. 2023. PMID: 37377258 Free PMC article. English, Portuguese. No abstract available.
-
Critical analysis of Chagas disease treatment in different countries.Mem Inst Oswaldo Cruz. 2022 Jul 8;117:e210034. doi: 10.1590/0074-02760210034. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35830002 Free PMC article. Review.
-
Benznidazole decreases the risk of chronic Chagas disease progression and cardiovascular events: A long-term follow up study.EClinicalMedicine. 2020 Dec 23;31:100694. doi: 10.1016/j.eclinm.2020.100694. eCollection 2021 Jan. EClinicalMedicine. 2020. PMID: 33554085 Free PMC article.
-
New chemotherapy regimens and biomarkers for Chagas disease: the rationale and design of the TESEO study, an open-label, randomised, prospective, phase-2 clinical trial in the Plurinational State of Bolivia.BMJ Open. 2021 Dec 31;11(12):e052897. doi: 10.1136/bmjopen-2021-052897. BMJ Open. 2021. PMID: 34972765 Free PMC article. Clinical Trial.
-
Pharmacokinetics of two pharmaceutical presentations of benznidazole in adult Trypanosoma cruzi infected population.Mem Inst Oswaldo Cruz. 2025 Feb 28;120:e240177. doi: 10.1590/0074-02760240177. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40052995 Free PMC article.
References
-
- Global Burden of Disease (GBD) for Chagas disease 2017 [December 28th 2019]. https://vizhub.healthdata.org/gbd-compare/.
-
- Hotez PJ, Bottazzi ME, Franco‐Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Neglected Tropical Diseases 2008;2(9):e300 10.1371/journal.pntd.0000300 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

