Spermathecal variation in temperate Opiliones
- PMID: 32805033
- PMCID: PMC10388384
- DOI: 10.1093/icb/icaa120
Spermathecal variation in temperate Opiliones
Abstract
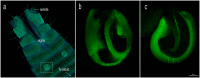
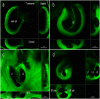
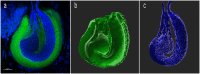
Most arachnid fertilization occurs internally, allowing for a variety of post-copulatory mechanisms to take place. Females are expected to exert some level of control over sperm fate when 1) the point of gametic fusion is particularly distant from the point of oogenesis, 2) the time of syngamy is significantly later than the time of mating, 3) sperm are non-motile, and/or 4) the morphology of females allows for selective containment of sperm. Many of these conditions are met in Opiliones (a.k.a. "harvesters," "harvestmen," or "daddy-longlegs"), where we have evidence of sexual antagonism, multiple mating, and delayed oviposition for a number of species. We used confocal laser scanning microscopy to capture and analyze images of harvester spermathecae, structures within the genitalia of female arthropods that store and maintain sperm after copulation. Spermathecal morphology may have critical function in controlling seminal movement. We anticipated that species with previously identified traits associated with sexual antagonism would also have thicker and/or relatively more complex spermathecae. We examined spermathecal morphology in thirteen species of Leiobunum and one species of Hadrobunus, which were collected from North America and Japan. Our results show that eight species had structures consisting of a single chamber with no or partial invagination, and the remainder had multiple cuticular invaginations producing 2-3 lumina within the spermathecae. Using phylogenetic multivariate comparative methods, we estimated a trend towards cross-correlation between conflict and spermathecal traits. Some, but not all, of the species with thicker, more complex spermathecae had morphological traits associated with sexual conflict (larger body size, thicker genital muscle). In conclusion, we discuss methods to elucidate spermathecal mechanism and sperm precedence in these species. Confocal microscopy allowed us to visualize internal structures difficult to interpret with two-dimensional brightfield microscopy, a technique that could be applied to the characterization of internal reproductive structures in other arthropods.
Keywords: Opiliones; Spermathecae; phylogenetic comparative methods; sexual conflict.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

