Neonatal Abstinence Syndrome Severity Index Predicts 18-Month Neurodevelopmental Outcome in Neonates Randomized to Morphine or Methadone
- PMID: 32805259
- PMCID: PMC7731918
- DOI: 10.1016/j.jpeds.2020.08.034
Neonatal Abstinence Syndrome Severity Index Predicts 18-Month Neurodevelopmental Outcome in Neonates Randomized to Morphine or Methadone
Abstract
Objective: To develop an index to determine which opioid-exposed neonates have the most severe neonatal abstinence syndrome (NAS).
Study design: Full-term neonates with NAS (n = 116) from mothers maintained on methadone or buprenorphine were enrolled from 8 sites into a randomized clinical trial of morphine vs methadone. Ninety-nine (85%) were evaluated at hospital discharge using the NICU Network Neurobehavioral Scale (NNNS). At 18 months, 83 of 99 (83.8%) were evaluated with the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), and 77 of 99 (77.7%) were evaluated with the Child Behavior Checklist (CBCL).
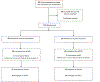
Results: Cluster analysis was used to define high (n = 21) and low (n = 77) NAS severity. Compared with infants in the low NAS severity cluster, infants in the high NAS severity cluster had a longer length of stay (P < .001), longer length of stay due to NAS (P < .001), longer duration of treatment due to NAS (P < .001), and higher total dose of the study drug (P < .001) and were more likely to have received phenobarbital (P < .001), to have been treated with morphine (P = .020), and to have an atypical NNNS profile (P = .005). The 2 groups did not differ in terms of maximum Finnegan score. At 18 months, in unadjusted analyses, compared with the high-severity cluster, the low-severity cluster had higher scores on the Bayley-III Cognitive (P = .013), Language (P < .001), and Motor (P = .041) composites and less total behavior problems on the CBCL (P = .028). In adjusted analyses, the difference in the Bayley-III Language composite remained (P = .013).
Conclusions: Presumptive measures of NAS severity can be aggregated to develop an index that predicts developmental outcomes at age 18 months.
Keywords: NAS; NAS severity; neonatal abstinence syndrome; neurodevelopmental outcome; prenatal opioid exposure.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Healthcare Cost and Utilization Project (H-CUP). Agency for Healthcare Research and Quality (AHRQ). Trends in neonatal abstinence syndrome births in the United States. 2018. https://www.hcup-us.ahrq.gov/reports/Trends_NeonatalAbstinenceSyndrome_B.... Accessed March 20, 2020.
-
- Holmes AV, Atwood EC, Whalen B, Beliveau J, Jarvis JD, Matulis JC, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics 2016;137:e20152929. - PubMed
-
- Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of neonatal abstinence syndrome—28 states, 1999–2013. MMWR Morb Mortal Wkly Rep 2016;65:799–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

