The Long Noncoding RNA CCAT2 Induces Chromosomal Instability Through BOP1-AURKB Signaling
- PMID: 32805281
- PMCID: PMC7725986
- DOI: 10.1053/j.gastro.2020.08.018
The Long Noncoding RNA CCAT2 Induces Chromosomal Instability Through BOP1-AURKB Signaling
Abstract
Background & aims: Chromosomal instability (CIN) is a carcinogenesis event that promotes metastasis and resistance to therapy by unclear mechanisms. Expression of the colon cancer-associated transcript 2 gene (CCAT2), which encodes a long noncoding RNA (lncRNA), associates with CIN, but little is known about how CCAT2 lncRNA regulates this cancer enabling characteristic.
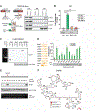
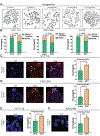
Methods: We performed cytogenetic analysis of colorectal cancer (CRC) cell lines (HCT116, KM12C/SM, and HT29) overexpressing CCAT2 and colon organoids from C57BL/6N mice with the CCAT2 transgene and without (controls). CRC cells were also analyzed by immunofluorescence microscopy, γ-H2AX, and senescence assays. CCAT2 transgene and control mice were given azoxymethane and dextran sulfate sodium to induce colon tumors. We performed gene expression array and mass spectrometry to detect downstream targets of CCAT2 lncRNA. We characterized interactions between CCAT2 with downstream proteins using MS2 pull-down, RNA immunoprecipitation, and selective 2'-hydroxyl acylation analyzed by primer extension analyses. Downstream proteins were overexpressed in CRC cells and analyzed for CIN. Gene expression levels were measured in CRC and non-tumor tissues from 5 cohorts, comprising more than 900 patients.
Results: High expression of CCAT2 induced CIN in CRC cell lines and increased resistance to 5-fluorouracil and oxaliplatin. Mice that expressed the CCAT2 transgene developed chromosome abnormalities, and colon organoids derived from crypt cells of these mice had a higher percentage of chromosome abnormalities compared with organoids from control mice. The transgenic mice given azoxymethane and dextran sulfate sodium developed more and larger colon polyps than control mice given these agents. Microarray analysis and mass spectrometry indicated that expression of CCAT2 increased expression of genes involved in ribosome biogenesis and protein synthesis. CCAT2 lncRNA interacted directly with and stabilized BOP1 ribosomal biogenesis factor (BOP1). CCAT2 also increased expression of MYC, which activated expression of BOP1. Overexpression of BOP1 in CRC cell lines resulted in chromosomal missegregation errors, and increased colony formation, and invasiveness, whereas BOP1 knockdown reduced viability. BOP1 promoted CIN by increasing the active form of aurora kinase B, which regulates chromosomal segregation. BOP1 was overexpressed in polyp tissues from CCAT2 transgenic mice compared with healthy tissue. CCAT2 lncRNA and BOP1 mRNA or protein were all increased in microsatellite stable tumors (characterized by CIN), but not in tumors with microsatellite instability compared with nontumor tissues. Increased levels of CCAT2 lncRNA and BOP1 mRNA correlated with each other and with shorter survival times of patients.
Conclusions: We found that overexpression of CCAT2 in colon cells promotes CIN and carcinogenesis by stabilizing and inducing expression of BOP1 an activator of aurora kinase B. Strategies to target this pathway might be developed for treatment of patients with microsatellite stable colorectal tumors.
Keywords: Aneuploidy; MSS; Noncoding RNA; Tumorigenesis.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet 2012;13:189–203. - PubMed
-
- Boveri M. Über Mitosen bei einseitiger Chromosomenbindung. Jenaische Zeitschrift für Naturwissenschaft 1903;37:401–443.
-
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol 2010;11:220–8. - PubMed
-
- Gronroos E, Lopez-Garcia C. Tolerance of Chromosomal Instability in Cancer: Mechanisms and Therapeutic Opportunities. Cancer Res 2018;78:6529–6535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM126942/GM/NIGMS NIH HHS/United States
- R01 CA202797/CA/NCI NIH HHS/United States
- U54 CA096297/CA/NCI NIH HHS/United States
- UH3 TR000943/TR/NCATS NIH HHS/United States
- R50 CA211121/CA/NCI NIH HHS/United States
- U54 CA096300/CA/NCI NIH HHS/United States
- R01 GM121487/GM/NIGMS NIH HHS/United States
- R01 CA072851/CA/NCI NIH HHS/United States
- R01 CA182905/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA222007/CA/NCI NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- R01 GM122775/GM/NIGMS NIH HHS/United States
- R01 CA181572/CA/NCI NIH HHS/United States
- R50 CA243707/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

