Evaluating UiO-66 Metal-Organic Framework Nanoparticles as Acid-Sensitive Carriers for Pulmonary Drug Delivery Applications
- PMID: 32805901
- PMCID: PMC7719435
- DOI: 10.1021/acsami.0c10900
Evaluating UiO-66 Metal-Organic Framework Nanoparticles as Acid-Sensitive Carriers for Pulmonary Drug Delivery Applications
Abstract
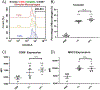
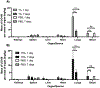
Developing novel drug carriers for pulmonary delivery is necessary to achieve higher efficacy and consistency for treating pulmonary diseases while limiting off-target side effects that occur from alternative routes of administration. Metal-organic frameworks (MOFs) have recently emerged as a class of materials with characteristics well-suited for pulmonary drug delivery, with chemical tunability, high surface area, and pore size, which will allow for efficient loading of therapeutic cargo and deep lung penetration. UiO-66, a zirconium and terephthalic acid-based MOF, has displayed notable chemical and physical stability and potential biocompatibility; however, its feasibility for use as a pulmonary drug delivery vehicle has yet to be examined. Here, we evaluate the use of UiO-66 nanoparticles (NPs) as novel pulmonary drug delivery vehicles and assess the role of missing linker defects in their utility for this application. We determined that missing linker defects result in differences in NP aerodynamics but have minimal effects on the loading of model and therapeutic cargo, cargo release, biocompatibility, or biodistribution. This is a critical result, as it indicates the robust consistency of UiO-66, a critical feature for pulmonary drug delivery, which is plagued by inconsistent dosage because of variable properties. Not only that, but UiO-66 NPs also demonstrate pH-dependent stability, with resistance to degradation in extracellular conditions and breakdown in intracellular environments. Furthermore, the carriers exhibit high biocompatibility and low cytotoxicity in vitro and are well-tolerated in in vivo murine evaluations of orotracheally administered NPs. Following pulmonary delivery, UiO-66 NPs remain localized to the lungs before clearance over the course of seven days. Our results demonstrate the feasibility of using UiO-66 NPs as a novel platform for pulmonary drug delivery through their tunable NP properties, which allow for controlled aerodynamics and internalization-dependent cargo release while displaying remarkable pulmonary biocompatibility.
Keywords: UiO-66; aerosols; defectiveness; metal−organic frameworks; nanoparticles; pulmonary drug delivery.
Conflict of interest statement
AUTHOR DISCLOSURE STATEMENT
No conflicts of interest exist.
Figures




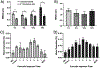
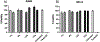




References
-
- Trickett CA; Helal A; Al-Maythalony BA; Yamani ZH; Cordova KE; Yaghi OM The Chemistry of Metal–Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater 2017, 2 (8), 17045 10.1038/natrevmats.2017.45. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

