Crystal Structure and Stoichiometric Composition of Potassium-Intercalated Tetracene
- PMID: 32805995
- PMCID: PMC7482393
- DOI: 10.1021/acs.inorgchem.0c01635
Crystal Structure and Stoichiometric Composition of Potassium-Intercalated Tetracene
Abstract
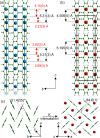
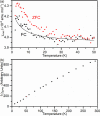
The products of the solid-state reactions between potassium metal and tetracene (K:Tetracene, 1:1, 1.5:1, and 2:1) are fully structurally characterized. Synchrotron X-ray powder diffraction shows that only K2Tetracene forms under the reaction conditions studied, with unreacted tetracene always present for x < 2. Diffraction and 13C MAS NMR show that K2Tetracene has a crystal structure that is analogous to that of K2Pentacene, but with the cations ordered on two sites because of the influence of the length of the hydrocarbon on possible cation positions. K2Tetracene is a nonmagnetic insulator, thus further questioning the nature of reported superconductivity in this class of materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Kubozono Y.; Mitamura H.; Lee X.; He X.; Yamanari Y.; Takahashi Y.; Suzuki Y.; Kaji Y.; Eguchi R.; Akaike K.; Kambe T.; Okamoto H.; Fujiwara A.; Kato T.; Kosugi T.; Aoki H. Metal-intercalated aromatic hydrocarbons: a new class of carbon-based superconductors. Phys. Chem. Chem. Phys. 2011, 13 (37), 16476–16493. 10.1039/c1cp20961b. - DOI - PubMed
-
- Haddon R. C.; Hebard A. F.; Rosseinsky M. J.; Murphy D. W.; Duclos S. J.; Lyons K. B.; Miller B.; Rosamilia J. M.; Fleming R. M.; Kortan A. R.; Glarum S. H.; Makhija A. V.; Muller A. J.; Eick R. H.; Zahurak S. M.; Tycko R.; Dabbagh G.; Thiel F. A. Conducting films of C60 and C70 by alkali-metal doping. Nature 1991, 350 (6316), 320–322. 10.1038/350320a0. - DOI
LinkOut - more resources
Full Text Sources

