The Influence of NaCl and Glucose Content on Growth and Ochratoxin A Production by Aspergillus ochraceus, Aspergillus carbonarius and Penicillium nordicum
- PMID: 32806492
- PMCID: PMC7472267
- DOI: 10.3390/toxins12080515
The Influence of NaCl and Glucose Content on Growth and Ochratoxin A Production by Aspergillus ochraceus, Aspergillus carbonarius and Penicillium nordicum
Abstract
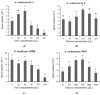
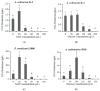
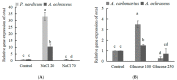
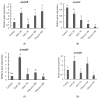
Ochratoxin A (OTA) is a nephrotoxic mycotoxin, which deserves particular attention for its widespread contamination of a variety of food and feed. Aspergillus ochraceus, Aspergillus carbonarius, and Penicillium nordicum are an important source of OTA in three different kinds of food commodities, including cereals, grape and dried fruit products, and dry-cured meat products. Deeper knowledge of OTA production and mycelium growth related to the high-sugar or NaCl-rich environments was gained in this manuscript. A. ochraceus and P. nordicum were likely to have greater growth rates in medium supplied with certain concentrations of NaCl (0-80 g/L), and the colony diameter was the largest at the salt content of 40 g/L. P. nordicum was more suitable to grow in NaCl-riched medium, the OTA production was increased to 316 ppb from 77 ppb when 20 g/L NaCl was added. The capability of OTA production was inhibited when salt content was 40 g/L and 60 g/L in A. ochraceus and P. nordicum, respectively. As the glucose content increased to 250 g/L, the capacity of mycelium growth and sporulation was increased significantly in A. ochraceus and A. carbonarius. A. carbonarius was more suitable to grow in high-sugar grape products. OTA production was significantly promoted with an added 100 g/L glucose in A. carbonarius. OTA production was inhibited when glucose content was 150 g/L and in 200 g/L in A. ochraceus and A. carbonarius, respectively. NaCl and glucose have an effect on fungal growth and OTA production, and the activation of biosynthetic genes of OtaA. These results would allow designing new strategies to prevent OTA accumulation on sugar or NaCl-riched foodstuffs and achieve the objective to manufacture cereals, dried vine fruits and dry-cured ham, free of OTA.
Keywords: A. carbonarius; A. ochraceus; NaCl-riched; P. nordicum; high-sugar; ochratoxin A.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Zhao T., Shen X.L., Chen W., Liao X., Yang J., Wang Y., Zou Y., Fang C. Advances in research of nephrotoxicity and toxic antagonism of Ochratoxin A. Toxin Rev. 2017;36:39–44. doi: 10.1080/15569543.2016.1243560. - DOI
-
- Ramos A.J., Labernia N., Marín S., Sanchis V., Magan N. Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int. J. Food Microbiol. 1998;44:133–140. doi: 10.1016/S0168-1605(98)00131-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

