Systemic Insulin Resistance and Metabolic Perturbations in Chow Fed Inducible Nitric Oxide Synthase Knockout Male Mice: Partial Reversal by Nitrite Supplementation
- PMID: 32806494
- PMCID: PMC7465804
- DOI: 10.3390/antiox9080736
Systemic Insulin Resistance and Metabolic Perturbations in Chow Fed Inducible Nitric Oxide Synthase Knockout Male Mice: Partial Reversal by Nitrite Supplementation
Abstract
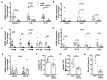
iNOS, an important mediator of inflammation, has emerged as an important metabolic regulator. There are conflicting observations on the incidence of insulin resistance (IR) due to hyperglycemia/dyslipidemia in iNOS-/- mice. There are reports that high fat diet (HFD) fed mice exhibited no change, protection, or enhanced susceptibility to IR. Similar observations were also reported for low fat diet (LFD) fed KO mice. In the present study chow fed iNOS-/- mice were examined for the incidence of IR, and metabolic perturbations, and also for the effect of sodium nitrite supplementation (50 mg/L). In IR-iNOS-/- mice, we observed significantly higher body weight, BMI, adiposity, blood glucose, HOMA-IR, serum/tissue lipids, glucose intolerance, enhanced gluconeogenesis, and disrupted insulin signaling. Expression of genes involved in hepatic and adipose tissue lipid uptake, synthesis, oxidation, and gluconeogenesis was upregulated with concomitant downregulation of genes for hepatic lipid excretion. Nitrite supplementation restored NO levels, significantly improved systemic IR, glucose tolerance, and also reduced lipid accumulation by rescuing hepatic insulin sensitivity, glucose, and lipid homeostasis. Obesity, gluconeogenesis, and adipose tissue insulin signaling were only partially reversed in nitrite supplemented iNOS-/- mice. Our results thus demonstrate that nitrite supplementation to iNOS-/- mice improves insulin sensitivity and metabolic homeostasis, thus further highlighting the metabolic role of iNOS.
Keywords: adipose tissue; dyslipidemia; iNOS−/−; insulin resistance; liver; metabolism; nitric oxide; nitrite.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- House L.M., Morris R.T., Barnes T.M., Lantier L., Cyphert T.J., McGuinness O.P., Otero Y.F. Tissue inflammation and nitric oxide-mediated alterations in cardiovascular function are major determinants of endotoxin-induced insulin resistance. Cardiovasc. Diabetol. 2015;14:56. doi: 10.1186/s12933-015-0223-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

