Quorum Sensing and Toxin Production in Staphylococcus aureus Osteomyelitis: Pathogenesis and Paradox
- PMID: 32806558
- PMCID: PMC7471978
- DOI: 10.3390/toxins12080516
Quorum Sensing and Toxin Production in Staphylococcus aureus Osteomyelitis: Pathogenesis and Paradox
Abstract
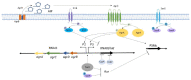
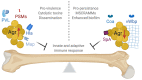
Staphylococcus aureus is a Gram-positive pathogen capable of infecting nearly every vertebrate organ. Among these tissues, invasive infection of bone (osteomyelitis) is particularly common and induces high morbidity. Treatment of osteomyelitis is notoriously difficult and often requires debridement of diseased bone in conjunction with prolonged antibiotic treatment to resolve infection. During osteomyelitis, S. aureus forms characteristic multicellular microcolonies in distinct niches within bone. Virulence and metabolic responses within these multicellular microcolonies are coordinated, in part, by quorum sensing via the accessory gene regulator (agr) locus, which allows staphylococcal populations to produce toxins and adapt in response to bacterial density. During osteomyelitis, the Agr system significantly contributes to dysregulation of skeletal homeostasis and disease severity but may also paradoxically inhibit persistence in the host. Moreover, the Agr system is subject to complex crosstalk with other S. aureus regulatory systems, including SaeRS and SrrAB, which can significantly impact the progression of osteomyelitis. The objective of this review is to highlight Agr regulation, its implications on toxin production, factors that affect Agr activation, and the potential paradoxical influences of Agr regulation on disease progression during osteomyelitis.
Keywords: Staphylococcus aureus; accessory gene regulator; bone; infectious; osteomyelitis; pathogenesis; quorum; toxin; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hatzenbuehler J., Pulling T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician. 2011;84:1027–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

