Neoadjuvant Radiotherapy-Related Wound Morbidity in Soft Tissue Sarcoma: Perspectives for Radioprotective Agents
- PMID: 32806601
- PMCID: PMC7465163
- DOI: 10.3390/cancers12082258
Neoadjuvant Radiotherapy-Related Wound Morbidity in Soft Tissue Sarcoma: Perspectives for Radioprotective Agents
Abstract
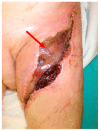
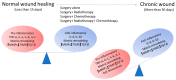
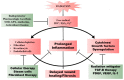
Historically, patients with localized soft tissue sarcomas (STS) of the extremities would undergo limb amputation. It was subsequently determined that the addition of radiation therapy (RT) delivered prior to (neoadjuvant) or after (adjuvant) a limb-sparing surgical resection yielded equivalent survival outcomes to amputation in appropriate patients. Generally, neoadjuvant radiation offers decreased volume and dose of high-intensity radiation to normal tissue and increased chance of achieving negative surgical margins-but also increases wound healing complications when compared to adjuvant radiotherapy. This review elaborates on the current neoadjuvant/adjuvant RT approaches, wound healing complications in STS, and the potential application of novel radioprotective agents to minimize radiation-induced normal tissue toxicity.
Keywords: limb preservation; neoadjuvant radiotherapy; radioprotective agents; radiotherapy complications; soft tissue sarcoma; wound healing.
Conflict of interest statement
The sponsors had no role in the design, execution, interpretation, or writing of this study. This review covers previous publications by some of its co-authors. No authors wrote, reviewed, or made editorial comments on their past work. Allen, Milhem, and Monga all report working on SARC032, a clinical trial involving pembrolizumab for sarcomas which is partially funded by Merck. Monga additionally reports Honoraria from Forma Therapeutics, a research grant from Amgen, and travel expenses from Deciphera, Glaxo-Smith-Klien. Milhem additionally reports funding in the past 2 years for unrelated research from Merck™. Allen, Callaghan, Mapuskar, and Spitz, and Petronek have received grant funding for research related to Pharmacologic Ascorbate. Allen, Mapsuskar, and Spitz, and Petronek and Hasibuzzaman have received funding and/or participated in research related to SOD mimetics. Allen, Mapuskar, and Spitz, and Petronek have received funding, fees, and/or participated in research with Galera, a producer of a SOD mimetic agent. Miller reports the Orthopaedic Research and Education Foundation grant for unrelated work. All other authors (Coleman, Steinbach, and Rodman) declare no conflict of interest.
Figures
References
-
- Stinson S.F., Delaney T.F., Greenberg J., Yang J.C., Lampert M.H., Hicks J.E., Venzon D., White D.E., Rosenberg S.A., Glatstein E.J. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int. J. Radiat. Oncol. 1991;21:1493–1499. doi: 10.1016/0360-3016(91)90324-W. - DOI - PubMed
-
- Wilson A., Davis A., Bell R., O’Sullivan B., Catton C., Madadi F., Kandel R., Fornasier V. Local control of soft tissue sarcoma of the extremity: The experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur. J. Cancer. 1994;30:746–751. doi: 10.1016/0959-8049(94)90286-0. - DOI - PubMed
-
- Yang J.C., E Chang A., Baker A.R., Sindelar W.F., Danforth D.N., Topalian S.L., Delaney T., Glatstein E., Steinberg S.M., Merino M.J., et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

