Comprehensive Fungal Community Analysis of House Dust Using Next-Generation Sequencing
- PMID: 32806670
- PMCID: PMC7460106
- DOI: 10.3390/ijerph17165842
Comprehensive Fungal Community Analysis of House Dust Using Next-Generation Sequencing
Abstract
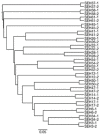
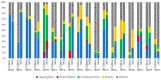
Fungal community analyses in homes have been attracting attention because fungi are now generally considered to be allergens. Currently, these analyses are generally conducted using the culture method, although fungal communities in households often contain species that are difficult to culture. In contrast, next-generation sequencing (NGS) represents a comprehensive, labor- and time-saving approach that can facilitate species identification. However, the reliability of the NGS method has not been compared to that of the culture method. In this study, in an attempt to demonstrate the reliability of this application, we used the NGS method to target the internal transcribed spacer 1 (ITS1) in the fungal genome, conducted fungal community analyses for 18 house-dust samples and analyzed fungal community structures. The NGS method positively correlated with the culture method regarding the relative abundance of Aspergillus, Penicillium, Cladosporium and yeasts, which represent the major fungal components found in houses. Furthermore, several genera, such as Malassezia, could be sensitively detected. Our results imply that the reliability of the NGS method is comparable to that of the culture method and indicates that easily available databases may require modifications, including the removal of registrations that have not been sufficiently classified at the genus level.
Keywords: ITS region; fungal community analysis; house dust; next-generation sequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

