Identification and Morphological Characterization of Biofilms Formed by Strains Causing Infection in Orthopedic Implants
- PMID: 32806685
- PMCID: PMC7460306
- DOI: 10.3390/pathogens9080649
Identification and Morphological Characterization of Biofilms Formed by Strains Causing Infection in Orthopedic Implants
Abstract
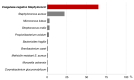
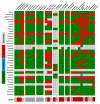
Objectives: For a better understanding of the mechanisms involved in biofilm formation, we performed a broad identification and characterization of the strains affecting implants by evaluating the morphology of biofilms formed in vitro in correlation with tests of the strains' antibiotic susceptibility in planktonic form. The ability of the strains to form biofilms in vitro was evaluated by means of colony forming units counting, metabolic activity tests of biofilm cells, and scanning electron microscopy. Methods: A total of 140 strains were isolated from patients with orthopedic implant-related infections during the period of 2015 to 2018. The identification of the isolates was carried out through microbiological cultures and confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Antibiotic susceptibility rates of the isolates were accessed according to EUCAST (European Committee on Antimicrobial Susceptibility Testing). The ability of all isolates to form biofilms in vitro was evaluated by counting the colony forming units, by measuring the metabolic activity of biofilm cells, and by analyzing the morphology of the formed biofilms using scanning electron microscopy. Results: From all the isolates, 41.84% (62 strains) were Staphylococcus epidermidis and 15.60% (22 strains) were Staphylococcus aureus. A significant difference in the capacity of biofilm formation was observed among the isolates. When correlating the biofilm forming capacity of the isolates to their antibiotic susceptibility rates, we observed that not all strains that were classified as resistant were biofilm producers in vitro. In other words, bacteria that are not good biofilm formers can show increased tolerance to multiple antibiotic substances. Conclusion: From 2015 until 2018, Staphylococcus epidermidis was the strain that caused most of the orthopedic implant-related infections in our hospital. Not all strains causing infection in orthopedic implants are able to form biofilms under in vitro conditions. Differences were observed in the number of cells and morphology of the biofilms. In addition, antibiotic resistance is not directly related to the capacity of the strains to form biofilms in vitro. Further studies should consider the use of in vitro culture conditions that better reproduce the joint environment and the growth of biofilms in humans.
Keywords: antibiotic susceptibility; biofilm; implant-related infections.; in vitro conditions.
Conflict of interest statement
The authors have no conflict of interest in relation to this study.
Figures






Similar articles
-
Does Extracellular DNA Production Vary in Staphylococcal Biofilms Isolated From Infected Implants versus Controls?Clin Orthop Relat Res. 2017 Aug;475(8):2105-2113. doi: 10.1007/s11999-017-5266-0. Epub 2017 Feb 13. Clin Orthop Relat Res. 2017. PMID: 28194715 Free PMC article.
-
Biofilm forming capacity and antibiotic susceptibility of Staphylococcus spp. with the icaA/icaD/bap genotype isolated from ocular surface of patients with diabetes.Malawi Med J. 2018 Dec;30(4):243-249. doi: 10.4314/mmj.v30i4.6. Malawi Med J. 2018. PMID: 31798802 Free PMC article.
-
Antibacterial and Anti-Biofilm Activity of Omega-3 Polyunsaturated Fatty Acids against Periprosthetic Joint Infections-Isolated Multi-Drug Resistant Strains.Biomedicines. 2021 Mar 26;9(4):334. doi: 10.3390/biomedicines9040334. Biomedicines. 2021. PMID: 33810261 Free PMC article.
-
Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI).Microorganisms. 2024 Jun 14;12(6):1198. doi: 10.3390/microorganisms12061198. Microorganisms. 2024. PMID: 38930580 Free PMC article. Review.
-
The Role of Staphylococcal Biofilm on the Surface of Implants in Orthopedic Infection.Microorganisms. 2022 Sep 26;10(10):1909. doi: 10.3390/microorganisms10101909. Microorganisms. 2022. PMID: 36296183 Free PMC article. Review.
Cited by
-
Lyophilized Human Bone Allograft as an Antibiotic Carrier: An In Vitro and In Vivo Study.Antibiotics (Basel). 2022 Jul 19;11(7):969. doi: 10.3390/antibiotics11070969. Antibiotics (Basel). 2022. PMID: 35884224 Free PMC article.
-
The antibacterial activity and therapeutic potential of the amphibian-derived peptide TB_KKG6K.mSphere. 2025 Jun 25;10(6):e0101624. doi: 10.1128/msphere.01016-24. Epub 2025 May 19. mSphere. 2025. PMID: 40387366 Free PMC article.
-
Antibiotic-Polyphosphate Nanocomplexes: A Promising System for Effective Biofilm Eradication.Int J Nanomedicine. 2024 Sep 18;19:9707-9725. doi: 10.2147/IJN.S473241. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39309185 Free PMC article.
-
Insights into the microbiological and virulence characteristics of bacteria in orthopaedic implant infections: A study from Pakistan.PLoS One. 2023 Oct 17;18(10):e0292956. doi: 10.1371/journal.pone.0292956. eCollection 2023. PLoS One. 2023. PMID: 37847701 Free PMC article.
-
Promising applications of D-amino acids in periprosthetic joint infection.Bone Res. 2023 Mar 10;11(1):14. doi: 10.1038/s41413-023-00254-z. Bone Res. 2023. PMID: 36894568 Free PMC article. Review.
References
-
- Vastag B. Knee replacement underused, says panel: Useful option when nonsurgical therapies fail. JAMA. 2004;291:413–414. - PubMed
-
- Saeed K., McLaren A.C., Schwarz E.M., Antoci V., Arnold W.V., Chen A.F., Clauss M., Esteban J., Gant V., Hendershot E., et al. 2018 International consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthop. Res. 2019;37:1007–1017. doi: 10.1002/jor.24229. - DOI - PubMed
-
- Anderson D.J., Engemann J.J., Harrell L.J., Carmeli Y., Reller L.B., Kaye K.S. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2006;50:1715–1720. doi: 10.1128/AAC.50.5.1715-1720.2006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

