Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise
- PMID: 32806696
- PMCID: PMC7564546
- DOI: 10.3390/vaccines8030451
Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise
Abstract
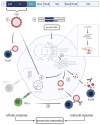
Tick-borne encephalitis virus (TBEV), a member of the family Flaviviridae, is one of the most important tick-transmitted viruses in Europe and Asia. Being a neurotropic virus, TBEV causes infection of the central nervous system, leading to various (permanent) neurological disorders summarized as tick-borne encephalitis (TBE). The incidence of TBE cases has increased due to the expansion of TBEV and its vectors. Since antiviral treatment is lacking, vaccination against TBEV is the most important protective measure. However, vaccination coverage is relatively low and immunogenicity of the currently available vaccines is limited, which may account for the vaccine failures that are observed. Understanding the TBEV-specific correlates of protection is of pivotal importance for developing novel and improved TBEV vaccines. For affording robust protection against infection and development of TBE, vaccines should induce both humoral and cellular immunity. In this review, the adaptive immunity induced upon TBEV infection and vaccination as well as novel approaches to produce improved TBEV vaccines are discussed.
Keywords: CD4+ T cells; CD8+ T cells; TBEV; antibodies; flavivirus; immunity; tick-borne encephalitis virus; vaccination; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organisation Vaccines against tick-borne encephalitis: WHO Position Paper. Wkly. Epidemiol. Rec. = Relev. Épidémiologique Hebd. 2011;86:241–256. - PubMed
-
- Ruzek D., Avšič Županc T., Borde J., Chrdle A., Eyer L., Karganova G., Kholodilov I., Knap N., Kozlovskaya L., Matveev A., et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019;164:23–51. doi: 10.1016/j.antiviral.2019.01.014. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

