Dermal Fibroblasts Internalize Phosphatidylserine-Exposed Secretory Melanosome Clusters and Apoptotic Melanocytes
- PMID: 32806720
- PMCID: PMC7461560
- DOI: 10.3390/ijms21165789
Dermal Fibroblasts Internalize Phosphatidylserine-Exposed Secretory Melanosome Clusters and Apoptotic Melanocytes
Abstract
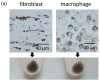
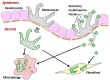
Pigmentation in the dermis is known to be caused by melanophages, defined as melanosome-laden macrophages. In this study, we show that dermal fibroblasts also have an ability to uptake melanosomes and apoptotic melanocytes. We have previously demonstrated that normal human melanocytes constantly secrete melanosome clusters from various sites of their dendrites. After adding secreted melanosome clusters collected from the culture medium of melanocytes, time-lapse imaging showed that fibroblasts actively attached to the secreted melanosome clusters and incorporated them. Annexin V staining revealed that phosphatidylserine (PtdSer), which is known as an 'eat-me' signal that triggers the internalization of apoptotic cells by macrophages, is exposed on the surface of secreted melanosome clusters. Dermal fibroblasts were able to uptake secreted melanosome clusters as did macrophages, and those fibroblasts express TIM4, a receptor for PtdSer-mediated endocytosis. Further, co-cultures of fibroblasts and melanocytes demonstrated that dermal fibroblasts internalize PtdSer-exposed apoptotic melanocytes. These results suggest that not only macrophages, but also dermal fibroblasts contribute to the collection of potentially toxic substances in the dermis, such as secreted melanosome clusters and apoptotic melanocytes, that have been occasionally observed to drop down into the dermis from the epidermis.
Keywords: apoptosis; dermis; epidermis; fibroblast; macrophage; melanin; melanocyte; melanosome; phosphatidylserine; pigmentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Braun-Falco O., Schoefinius H.H. Lentigo senilis. Hautarzt. 1971;22:277–283. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

