Exogenous Jaagsiekte Sheep Retrovirus type 2 (exJSRV2) related to ovine pulmonary adenocarcinoma (OPA) in Romania: prevalence, anatomical forms, pathological description, immunophenotyping and virus identification
- PMID: 32807166
- PMCID: PMC7433209
- DOI: 10.1186/s12917-020-02521-1
Exogenous Jaagsiekte Sheep Retrovirus type 2 (exJSRV2) related to ovine pulmonary adenocarcinoma (OPA) in Romania: prevalence, anatomical forms, pathological description, immunophenotyping and virus identification
Abstract
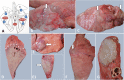
Background: Ovine pulmonary adenocarcinoma (OPA) is a neoplastic disease caused by exogenous Jaagsiekte Sheep Retrovirus (exJSRV). The prevalence of JSRV-related OPA in Eastern European countries, including Romania is unknown. We aimed to investigate: the prevalence and morphological features of OPA (classical and atypical forms) in the Transylvania region (Romania), the immunophenotype of the pulmonary tumors and their relationships with exJSRV infection. A total of 2693 adult ewes slaughtered between 2017 and 2019 in two private slaughterhouses from Transylvania region (Romania) was evaluated. Lung tumors were subsequently assessed by cytology, histology, immunocytochemistry, immunohistochemistry, electron microscopy and DNA testing.
Results: Out of 2693 examined sheep, 34 had OPA (1.26% prevalence). The diaphragmatic lobes were the most affected. Grossly, the classical OPA was identified in 88.24% of investigated cases and the atypical OPA in 11.76% that included solitary myxomatous nodules. Histopathology results confirmed the presence of OPA in all suspected cases, which were classified into acinar and papillary types. Myxoid growths (MGs) were diagnosed in 6 classical OPA cases and in 2 cases of atypical form. Lung adenocarcinoma was positive for MCK and TTF-1, and MGs showed immunoreaction for Vimentin, Desmin and SMA; Ki67 expression of classical OPA was higher than atypical OPA and MGs. JSRV-MA was identified by IHC (94.11%) in both epithelial and mesenchymal cells of OPA. Immunocytochemistry and electron microscopy also confirmed the JSRV within the neoplastic cells. ExJSRV was identified by PCR in 97.05% of analyzed samples. Phylogenetic analysis revealed the presence of the exJSRV type 2 (MT809678.1) in Romanian sheep affected by lung cancer and showed a high similarity with the UK strain (AF105220.1).
Conclusions: In this study, we confirmed for the first time in Romania the presence of exJSRV in naturally occurring OPA in sheep. Additionally, we described the first report of atypical OPA in Romania, and to the best of our knowledge, in Eastern Europe. Finally, we showed that MGs have a myofibroblastic origin.
Keywords: Atypical OPA; Epidemiology; Jaagsiekte sheep retrovirus type 2; Myxoid growths; Ovine pulmonary adenocarcinoma.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



References
-
- York D, Querat G. A history of ovine pulmonary adenocarcinoma (Jaagsiekte) and experiments leading to the deduction of the JSRV nucleotide sequence. In: Fan H, editor. Jaagsiekte sheep retrovirus and lung Cancer, Curr top in Microbiol Immunol. Berlin: Springer; 2003. pp. 1–25. - PubMed
-
- García-Goti M, González L, Cousens C, Cortabarría N, Extramiana AB, Minguijón E, Ortín A, De las Heras M, Sharp JM. Sheep pulmonary adenomatosis: characterization of two pathological forms associated with jaagsiekte retrovirus. J Comp Pathol. 2000;122:55–65. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

