A narrative review on trans-nasal pulmonary aerosol delivery
- PMID: 32807226
- PMCID: PMC7430014
- DOI: 10.1186/s13054-020-03206-9
A narrative review on trans-nasal pulmonary aerosol delivery
Abstract
The use of trans-nasal pulmonary aerosol delivery via high-flow nasal cannula (HFNC) has expanded in recent years. However, various factors influencing aerosol delivery in this setting have not been precisely defined, and no consensus has emerged regarding the optimal techniques for aerosol delivery with HFNC. Based on a comprehensive literature search, we reviewed studies that assessed trans-nasal pulmonary aerosol delivery with HFNC by in vitro experiments, and in vivo, by radiolabeled, pharmacokinetic and pharmacodynamic studies. In these investigations, the type of nebulizer employed and its placement, carrier gas, the relationship between gas flow and patient's inspiratory flow, aerosol delivery strategies (intermittent unit dose vs continuous administration by infusion pump), and open vs closed mouth breathing influenced aerosol delivery. The objective of this review was to provide rational recommendations for optimizing aerosol delivery with HFNC in various clinical settings.
Keywords: Aerosol therapy; Asthma; Chronic obstructive pulmonary disease; High-flow nasal cannula; Jet nebulizer; Oxygen therapy; Pulmonary hypertension; Vibrating mesh nebulizer.
Conflict of interest statement
Dr. Dhand reports remuneration from GSK Pharmaceuticals, Boehringer-Ingelheim, Bayer, Mylan, Teva, and Astra-Zeneca Pharmaceuticals outside the submitted work. Dr. Fink is Chief Science Officer for Aerogen Pharma Corp, San Mateo, CA, USA. Dr. MacLoughlin is the Senior Manager Science for Aerogen Ltd., Galway, Ireland. Dr. Li declares to receive research funding from Fisher & Paykel Healthcare, The Daniel and Ada Rice Foundation. None of the companies had a role in the conception of this review, in the literature search or interpretation, in the writing of the manuscript, or in the decision to publish the results.
Figures
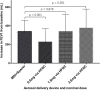

References
-
- Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–572. - PubMed
-
- Li J, Jing GQ, Scott JB. Year in review 2019: high-flow nasal cannula (HFNC) oxygen therapy for adult patients. Respir Care. 2020;65(4):545–557. - PubMed
-
- Kang H, Zhao Z, Tong Z. Effect of high-flow nasal cannula oxygen therapy in immunocompromised subjects with acute respiratory failure. Respir Care. 2020;65(3):369–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

