Vulnerability of progeroid smooth muscle cells to biomechanical forces is mediated by MMP13
- PMID: 32807790
- PMCID: PMC7431909
- DOI: 10.1038/s41467-020-17901-2
Vulnerability of progeroid smooth muscle cells to biomechanical forces is mediated by MMP13
Abstract
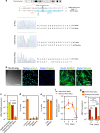
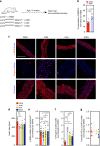
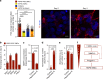
Hutchinson-Gilford Progeria Syndrome (HGPS) is a premature aging disease in children that leads to early death. Smooth muscle cells (SMCs) are the most affected cells in HGPS individuals, although the reason for such vulnerability remains poorly understood. In this work, we develop a microfluidic chip formed by HGPS-SMCs generated from induced pluripotent stem cells (iPSCs), to study their vulnerability to flow shear stress. HGPS-iPSC SMCs cultured under arterial flow conditions detach from the chip after a few days of culture; this process is mediated by the upregulation of metalloprotease 13 (MMP13). Importantly, double-mutant LmnaG609G/G609GMmp13-/- mice or LmnaG609G/G609GMmp13+/+ mice treated with a MMP inhibitor show lower SMC loss in the aortic arch than controls. MMP13 upregulation appears to be mediated, at least in part, by the upregulation of glycocalyx. Our HGPS-SMCs chip represents a platform for developing treatments for HGPS individuals that may complement previous pre-clinical and clinical treatments.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A. 2006;140:2603–2624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

