OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation
- PMID: 32807793
- PMCID: PMC7431428
- DOI: 10.1038/s41467-020-17885-z
OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation
Abstract
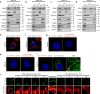
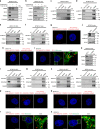
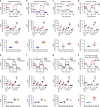
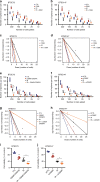
Glioblastoma contains a rare population of self-renewing brain tumor stem cells (BTSCs) which are endowed with properties to proliferate, spur the growth of new tumors, and at the same time, evade ionizing radiation (IR) and chemotherapy. However, the drivers of BTSC resistance to therapy remain unknown. The cytokine receptor for oncostatin M (OSMR) regulates BTSC proliferation and glioblastoma tumorigenesis. Here, we report our discovery of a mitochondrial OSMR that confers resistance to IR via regulation of oxidative phosphorylation, independent of its role in cell proliferation. Mechanistically, OSMR is targeted to the mitochondrial matrix via the presequence translocase-associated motor complex components, mtHSP70 and TIM44. OSMR interacts with NADH ubiquinone oxidoreductase 1/2 (NDUFS1/2) of complex I and promotes mitochondrial respiration. Deletion of OSMR impairs spare respiratory capacity, increases reactive oxygen species, and sensitizes BTSCs to IR-induced cell death. Importantly, suppression of OSMR improves glioblastoma response to IR and prolongs lifespan.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Transcriptional control of brain tumor stem cells by a carbohydrate binding protein.Cell Rep. 2021 Aug 31;36(9):109647. doi: 10.1016/j.celrep.2021.109647. Cell Rep. 2021. PMID: 34469737
-
Annexin A2-STAT3-Oncostatin M receptor axis drives phenotypic and mesenchymal changes in glioblastoma.Acta Neuropathol Commun. 2020 Apr 5;8(1):42. doi: 10.1186/s40478-020-00916-7. Acta Neuropathol Commun. 2020. PMID: 32248843 Free PMC article.
-
Control of glioblastoma tumorigenesis by feed-forward cytokine signaling.Nat Neurosci. 2016 Jun;19(6):798-806. doi: 10.1038/nn.4295. Epub 2016 Apr 25. Nat Neurosci. 2016. PMID: 27110918 Free PMC article.
-
Targeting glioblastoma with a brain-penetrant drug that impairs brain tumor stem cells via NLE1-Notch1 complex.Stem Cell Reports. 2024 Nov 12;19(11):1534-1547. doi: 10.1016/j.stemcr.2024.09.007. Epub 2024 Oct 17. Stem Cell Reports. 2024. PMID: 39423824 Free PMC article.
-
Anti-oncostatin M antibody inhibits the pro-malignant effects of oncostatin M receptor overexpression in squamous cell carcinoma.J Pathol. 2018 Mar;244(3):283-295. doi: 10.1002/path.5010. Epub 2018 Jan 9. J Pathol. 2018. PMID: 29205362
Cited by
-
Development and Validation of a Robust Immune-Related Prognostic Signature for Gastric Cancer.J Immunol Res. 2021 Apr 30;2021:5554342. doi: 10.1155/2021/5554342. eCollection 2021. J Immunol Res. 2021. PMID: 34007851 Free PMC article.
-
Deciphering the Biological Effects of Radiotherapy in Cancer Cells.Biomolecules. 2022 Aug 23;12(9):1167. doi: 10.3390/biom12091167. Biomolecules. 2022. PMID: 36139006 Free PMC article. Review.
-
LIN28B and Let-7 in Diffuse Midline Glioma: A Review.Cancers (Basel). 2023 Jun 19;15(12):3241. doi: 10.3390/cancers15123241. Cancers (Basel). 2023. PMID: 37370851 Free PMC article. Review.
-
Oncostatin-M and OSM-Receptor Feed-Forward Activation of MAPK Induces Separable Stem-like and Mesenchymal Programs.Mol Cancer Res. 2023 Sep 1;21(9):975-990. doi: 10.1158/1541-7786.MCR-22-0715. Mol Cancer Res. 2023. PMID: 37310811 Free PMC article.
-
Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells.Adv Sci (Weinh). 2025 May;12(20):e2412505. doi: 10.1002/advs.202412505. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285641 Free PMC article.
References
-
- Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Louis DN, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. - PubMed
-
- Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

