Methylglyoxal inhibits nuclear division through alterations in vacuolar morphology and accumulation of Atg18 on the vacuolar membrane in Saccharomyces cerevisiae
- PMID: 32807835
- PMCID: PMC7431575
- DOI: 10.1038/s41598-020-70802-8
Methylglyoxal inhibits nuclear division through alterations in vacuolar morphology and accumulation of Atg18 on the vacuolar membrane in Saccharomyces cerevisiae
Abstract
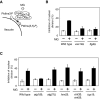
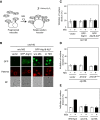
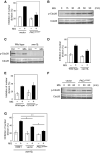
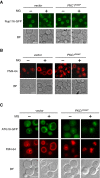
Methylglyoxal (MG) is a natural metabolite derived from glycolysis, and it inhibits the growth of cells in all kinds of organisms. We recently reported that MG inhibits nuclear division in Saccharomyces cerevisiae. However, the mechanism by which MG blocks nuclear division remains unclear. Here, we show that increase in the levels of phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) is crucial for the inhibitory effects of MG on nuclear division, and the deletion of PtdIns(3,5)P2-effector Atg18 alleviated the MG-mediated inhibitory effects. Previously, we reported that MG altered morphology of the vacuole to a single swelling form, where PtdIns(3,5)P2 accumulates. The changes in the vacuolar morphology were also needed by MG to exert its inhibitory effects on nuclear division. The known checkpoint machinery, including the spindle assembly checkpoint and morphological checkpoint, are not involved in the blockade of nuclear division by MG. Our results suggest that both the accumulation of Atg18 on the vacuolar membrane and alterations in vacuolar morphology are necessary for the MG-induced inhibition of nuclear division.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cavanaugh AM, Jaspersen SL. Big lessons from little yeast: Budding and fission yeast centrosome structure, duplication, and function. Annu. Rev. Genet. 2017;51:361–383. - PubMed
-
- Cid VJ, et al. Orchestrating the cell cycle in yeast: sequential localization of key mitotic regulators at the spindle pole and the bud neck. Microbiology. 2002;148:2647–2659. - PubMed
-
- Inoue Y, Kimura A. Methylglyoxal and regulation of its metabolism in microorganisms. Adv. Microb. Physiol. 1995;37:177–227. - PubMed
-
- Inoue Y, Maeta K, Nomura W. Glyoxalase system in yeasts: structure, function, and physiology. Semin. Cell. Dev. Biol. 2011;22:278–284. - PubMed
-
- Zemva J, et al. Effects of the reactive metabolite methylglyoxal on cellular signalling, insulin action and metabolism—What we know in mammals and what we can learn from yeast. Exp. Clin. Endocrinol. Diabetes. 2019;127:203–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

