Atomic force microscopy for revealing micro/nanoscale mechanics in tumor metastasis: from single cells to microenvironmental cues
- PMID: 32807839
- PMCID: PMC8027022
- DOI: 10.1038/s41401-020-0494-3
Atomic force microscopy for revealing micro/nanoscale mechanics in tumor metastasis: from single cells to microenvironmental cues
Abstract
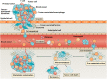
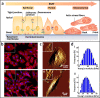
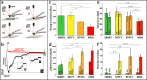
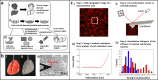
Mechanics are intrinsic properties which appears throughout the formation, development, and aging processes of biological systems. Mechanics have been shown to play important roles in regulating the development and metastasis of tumors, and understanding tumor mechanics has emerged as a promising way to reveal the underlying mechanisms guiding tumor behaviors. In particular, tumors are highly complex diseases associated with multifaceted factors, including alterations in cancerous cells, tissues, and organs as well as microenvironmental cues, indicating that investigating tumor mechanics on multiple levels is significantly helpful for comprehensively understanding the effects of mechanics on tumor progression. Recently, diverse techniques have been developed for probing the mechanics of tumors, among which atomic force microscopy (AFM) has appeared as an excellent platform enabling simultaneously characterizing the structures and mechanical properties of living biological systems ranging from individual molecules and cells to tissue samples with unprecedented spatiotemporal resolution, offering novel possibilities for understanding tumor physics and contributing much to the studies of cancer. In this review, we survey the recent progress that has been achieved with the use of AFM for revealing micro/nanoscale mechanics in tumor development and metastasis. Challenges and future progress are also discussed.
Keywords: atomic force microscopy; cancerous cell; exosome; extracellular matrix; tumor mechanics; tumor microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Mapping of biomechanical properties of cell lines on altered matrix stiffness using atomic force microscopy.Biomech Model Mechanobiol. 2020 Oct;19(5):1523-1536. doi: 10.1007/s10237-019-01285-4. Epub 2020 Jan 6. Biomech Model Mechanobiol. 2020. PMID: 31907681
-
Harnessing atomic force microscopy-based single-cell analysis to advance physical oncology.Microsc Res Tech. 2024 Apr;87(4):631-659. doi: 10.1002/jemt.24467. Epub 2023 Dec 6. Microsc Res Tech. 2024. PMID: 38053519 Review.
-
Single-cell mechanics--An experimental-computational method for quantifying the membrane-cytoskeleton elasticity of cells.Acta Biomater. 2015 Nov;27:224-235. doi: 10.1016/j.actbio.2015.08.028. Epub 2015 Aug 20. Acta Biomater. 2015. PMID: 26300334
-
Force nanoscopy of cell mechanics and cell adhesion.Nanoscale. 2013 May 21;5(10):4094-104. doi: 10.1039/c3nr00340j. Nanoscale. 2013. PMID: 23535827 Review.
-
Atomic Force Microscopy in Characterizing Cell Mechanics for Biomedical Applications: A Review.IEEE Trans Nanobioscience. 2017 Sep;16(6):523-540. doi: 10.1109/TNB.2017.2714462. Epub 2017 Jun 9. IEEE Trans Nanobioscience. 2017. PMID: 28613180 Review.
Cited by
-
Advances in Extracellular Matrix-Associated Diagnostics and Therapeutics.J Clin Med. 2025 Mar 10;14(6):1856. doi: 10.3390/jcm14061856. J Clin Med. 2025. PMID: 40142664 Free PMC article. Review.
-
Mechanics and disease of heart cells/cardiomyocytes explored through atomic force microscopy: present and future.Biophys Rev. 2025 Apr 9;17(2):347-358. doi: 10.1007/s12551-025-01307-9. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376399 Review.
-
Molecular Profiling of Liquid Biopsies for Precision Oncology.Adv Exp Med Biol. 2022;1361:235-247. doi: 10.1007/978-3-030-91836-1_13. Adv Exp Med Biol. 2022. PMID: 35230692 Review.
-
AFM Force Relaxation Curve Reveals That the Decrease of Membrane Tension Is the Essential Reason for the Softening of Cancer Cells.Front Cell Dev Biol. 2021 May 12;9:663021. doi: 10.3389/fcell.2021.663021. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34055793 Free PMC article.
-
Mechanical Studies of the Third Dimension in Cancer: From 2D to 3D Model.Int J Mol Sci. 2021 Sep 18;22(18):10098. doi: 10.3390/ijms221810098. Int J Mol Sci. 2021. PMID: 34576261 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, Vecchia CL, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28:1117–23.. - PubMed
-
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, Vecchia CL, et al. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol. 2018;29:1016–22.. - PubMed
-
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, Vecchia CL, et al. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann Oncol. 2019;30:781–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

