High levels of ubidecarenone (oxidized CoQ10) delivered using a drug-lipid conjugate nanodispersion (BPM31510) differentially affect redox status and growth in malignant glioma versus non-tumor cells
- PMID: 32807842
- PMCID: PMC7431533
- DOI: 10.1038/s41598-020-70969-0
High levels of ubidecarenone (oxidized CoQ10) delivered using a drug-lipid conjugate nanodispersion (BPM31510) differentially affect redox status and growth in malignant glioma versus non-tumor cells
Abstract
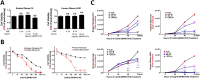
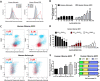
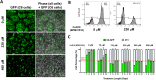
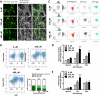
Metabolic reprogramming in cancer cells, vs. non-cancer cells, elevates levels of reactive oxygen species (ROS) leading to higher oxidative stress. The elevated ROS levels suggest a vulnerability to excess prooxidant loads leading to selective cell death, a therapeutically exploitable difference. Co-enzyme Q10 (CoQ10) an endogenous mitochondrial resident molecule, plays an important role in mitochondrial redox homeostasis, membrane integrity, and energy production. BPM31510 is a lipid-drug conjugate nanodispersion specifically formulated for delivery of supraphysiological concentrations of ubidecarenone (oxidized CoQ10) to the cell and mitochondria, in both in vitro and in vivo model systems. In this study, we sought to investigate the therapeutic potential of ubidecarenone in the highly treatment-refractory glioblastoma. Rodent (C6) and human (U251) glioma cell lines, and non-tumor human astrocytes (HA) and rodent NIH3T3 fibroblast cell lines were utilized for experiments. Tumor cell lines exhibited a marked increase in sensitivity to ubidecarenone vs. non-tumor cell lines. Further, elevated mitochondrial superoxide production was noted in tumor cells vs. non-tumor cells hours before any changes in proliferation or the cell cycle could be detected. In vitro co-culture experiments show ubidecarenone differentially affecting tumor cells vs. non-tumor cells, resulting in an equilibrated culture. In vivo activity in a highly aggressive orthotopic C6 glioma model demonstrated a greater than 25% long-term survival rate. Based on these findings we conclude that high levels of ubidecarenone delivered using BPM31510 provide an effective therapeutic modality targeting cancer-specific modulation of redox mechanisms for anti-cancer effects.
Conflict of interest statement
Jiaxin Sun, Taichang Jang, Milton Merchant, Seema Nagpal and Lawrence Recht received research support from both Brain Tumor Research fund and BERG.LLC. Shiva Kazerounian, Anne R. Diers, Michael A. Kiebish, Vivek K. Vishnudas, Stephane Gesta, Rangaprasad Sarangarajan and Niven R. Narain are all employed by BERG.LLC. Chirag B. Patel and Chen Chen have no competing interest to declare.
Figures


Similar articles
-
Elevated levels of mitochondrial CoQ10 induce ROS-mediated apoptosis in pancreatic cancer.Sci Rep. 2021 Mar 11;11(1):5749. doi: 10.1038/s41598-021-84852-z. Sci Rep. 2021. PMID: 33707480 Free PMC article.
-
Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth.J Biomed Nanotechnol. 2013 Mar;9(3):516-26. doi: 10.1166/jbn.2013.1547. J Biomed Nanotechnol. 2013. PMID: 23621009
-
Coenzyme Q10 protects astrocytes from ROS-induced damage through inhibition of mitochondria-mediated cell death pathway.Int J Biol Sci. 2015 Jan 1;11(1):59-66. doi: 10.7150/ijbs.10174. eCollection 2015. Int J Biol Sci. 2015. PMID: 25552930 Free PMC article.
-
Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury.Environ Toxicol. 2018 Feb;33(2):167-177. doi: 10.1002/tox.22505. Epub 2017 Nov 16. Environ Toxicol. 2018. PMID: 29143438
-
Therapeutic Potential of Co-enzyme Q10 in Retinal Diseases.Curr Med Chem. 2017;24(39):4329-4339. doi: 10.2174/0929867324666170801100516. Curr Med Chem. 2017. PMID: 28762311 Review.
Cited by
-
Cancer pharmacoinformatics: Databases and analytical tools.Funct Integr Genomics. 2024 Sep 19;24(5):166. doi: 10.1007/s10142-024-01445-5. Funct Integr Genomics. 2024. PMID: 39294509 Review.
-
Antioxidants in brain tumors: current therapeutic significance and future prospects.Mol Cancer. 2022 Oct 28;21(1):204. doi: 10.1186/s12943-022-01668-9. Mol Cancer. 2022. PMID: 36307808 Free PMC article. Review.
-
From signalling pathways to targeted therapies: unravelling glioblastoma's secrets and harnessing two decades of progress.Signal Transduct Target Ther. 2023 Oct 20;8(1):400. doi: 10.1038/s41392-023-01637-8. Signal Transduct Target Ther. 2023. PMID: 37857607 Free PMC article. Review.
-
Auxiliary effect of trolox on coenzyme Q10 restricts angiogenesis and proliferation of retinoblastoma cells via the ERK/Akt pathway.Sci Rep. 2024 Nov 9;14(1):27309. doi: 10.1038/s41598-024-76135-0. Sci Rep. 2024. PMID: 39516493 Free PMC article.
-
Exploring potential additive effects of 5-fluorouracil, thymoquinone, and coenzyme Q10 triple therapy on colon cancer cells in relation to glycolysis and redox status modulation.J Egypt Natl Canc Inst. 2025 Mar 10;37(1):7. doi: 10.1186/s43046-025-00261-7. J Egypt Natl Canc Inst. 2025. PMID: 40059278
References
-
- Warburg O. The metabolism of tumours. London: Arnold Constable; 1930.
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

