Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition
- PMID: 32807943
- PMCID: PMC9357342
- DOI: 10.1038/s41590-020-0753-y
Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition
Abstract
Type 2 cytokine responses promote parasitic immunity and initiate tissue repair; however, they can also result in immunopathologies when not properly restricted. Although basophilia is recognized as a common feature of type 2 inflammation, the roles basophils play in regulating these responses are unknown. Here, we demonstrate that helminth-induced group 2 innate lymphoid cell (ILC2) responses are exaggerated in the absence of basophils, resulting in increased inflammation and diminished lung function. Additionally, we show that ILC2s from basophil-depleted mice express reduced amounts of the receptor for the neuropeptide neuromedin B (NMB). Critically, NMB stimulation inhibited ILC2 responses from control but not basophil-depleted mice, and basophils were sufficient to directly enhance NMB receptor expression on ILC2s. These studies suggest that basophils prime ILC2s to respond to neuron-derived signals necessary to maintain tissue integrity. Further, these data provide mechanistic insight into the functions of basophils and identify NMB as a potent inhibitor of type 2 inflammation.
Conflict of interest statement
Competing Interests Statement
Mark C. Siracusa is the founder and president of NemaGen Discoveries.
Figures


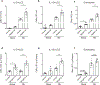





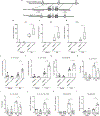



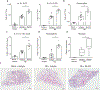





Comment in
-
Basophils balance ILC2s.Nat Rev Immunol. 2020 Oct;20(10):592-593. doi: 10.1038/s41577-020-00437-3. Nat Rev Immunol. 2020. PMID: 32807864 No abstract available.
References
-
- Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec 91, 585–595 (2016). - PubMed
Methods-only references
-
- Ohmori K et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. Journal of immunology (Baltimore, Md. : 1950) 182, 2835–2841, doi: 10.4049/jimmunol.0802870 (2009). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

