Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans
- PMID: 32807948
- PMCID: PMC8015416
- DOI: 10.1038/s41593-020-0692-9
Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans
Erratum in
-
Publisher Correction: Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans.Nat Neurosci. 2022 Sep;25(9):1247. doi: 10.1038/s41593-022-01155-w. Nat Neurosci. 2022. PMID: 35945454 No abstract available.
Abstract
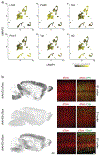
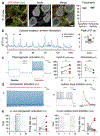
Recent success in identifying gene-regulatory elements in the context of recombinant adeno-associated virus vectors has enabled cell-type-restricted gene expression. However, within the cerebral cortex these tools are largely limited to broad classes of neurons. To overcome this limitation, we developed a strategy that led to the identification of multiple new enhancers to target functionally distinct neuronal subtypes. By investigating the regulatory landscape of the disease gene Scn1a, we discovered enhancers selective for parvalbumin (PV) and vasoactive intestinal peptide-expressing interneurons. Demonstrating the functional utility of these elements, we show that the PV-specific enhancer allowed for the selective targeting and manipulation of these neurons across vertebrate species, including humans. Finally, we demonstrate that our selection method is generalizable and characterizes additional PV-specific enhancers with exquisite specificity within distinct brain regions. Altogether, these viral tools can be used for cell-type-specific circuit manipulation and hold considerable promise for use in therapeutic interventions.
Conflict of interest statement
COMPETING INTEREST STATEMENT
The authors declare competing financial interests: The Broad Institute of MIT and Harvard has filed patent applications related to this work with Gord Fishell and Jordane Dimidschstein listed as inventors.
Figures







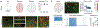

Comment in
-
Getting a Foot IN the Door: GABAergic INterneuron-Specific Enhancers.Epilepsy Curr. 2021 Mar;21(2):114-116. doi: 10.1177/1535759720985841. Epub 2021 Jan 7. Epilepsy Curr. 2021. PMID: 33412946 Free PMC article.
References
-
- Camp JG, Platt R & Treutlein B Mapping human cell phenotypes to genotypes with single-cell genomics. Science 365, 1401–1405 (2019). - PubMed
-
- Bedbrook CN, Deverman BE & Gradinaru V Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Annu. Rev. Neurosci 41, 323–348 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

