Targeted glycan degradation potentiates the anticancer immune response in vivo
- PMID: 32807964
- PMCID: PMC7727925
- DOI: 10.1038/s41589-020-0622-x
Targeted glycan degradation potentiates the anticancer immune response in vivo
Abstract
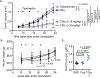
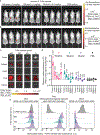
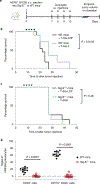
Currently approved immune checkpoint inhibitor therapies targeting the PD-1 and CTLA-4 receptor pathways are powerful treatment options for certain cancers; however, most patients across cancer types still fail to respond. Consequently, there is interest in discovering and blocking alternative pathways that mediate immune suppression. One such mechanism is an upregulation of sialoglycans in malignancy, which has been recently shown to inhibit immune cell activation through multiple mechanisms and therefore represents a targetable glycoimmune checkpoint. Since these glycans are not canonically druggable, we designed an αHER2 antibody-sialidase conjugate that potently and selectively strips diverse sialoglycans from breast cancer cells. In syngeneic breast cancer models, desialylation enhanced immune cell infiltration and activation and prolonged the survival of mice, an effect that was dependent on expression of the Siglec-E checkpoint receptor found on tumor-infiltrating myeloid cells. Thus, antibody-sialidase conjugates represent a promising modality for glycoimmune checkpoint therapy.
Figures













Comment in
-
Targeted self-destruction.Nat Chem Biol. 2020 Dec;16(12):1281-1283. doi: 10.1038/s41589-020-0612-z. Nat Chem Biol. 2020. PMID: 32807963 No abstract available.
References
-
- Ribas A et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. J. Am. Med. Assoc 315, 1600–1609 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

