Chemical syntheses of the salvinorin chemotype of KOR agonist
- PMID: 32808003
- PMCID: PMC7677207
- DOI: 10.1039/d0np00028k
Chemical syntheses of the salvinorin chemotype of KOR agonist
Abstract
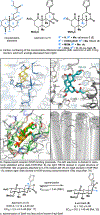
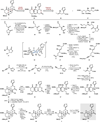
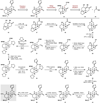
Covering: 2000 to 2020 The hallucinogenic diterpene salvinorin A potently and selectively agonizes the human kappa-opioid receptor (KOR). Its unique attributes-lack of a basic nitrogen, rapid brain penetrance, short half-life-combined with the potential of KOR as an emerging target for analgesics have stimulated extensive medicinal chemistry based on semi-synthesis from extracts of Salvia divinorum. Total synthesis efforts have delivered multiple, orthogonal routes to salvinorin A, its congeners and related analogs with the goal of optimizing its activity towards multiple functional endpoints. Here we review total syntheses of the salvinorin chemotype and discuss outstanding problems that synthesis can address in the future.
Figures

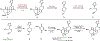

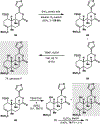

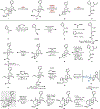


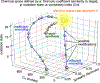

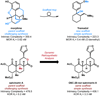
References
-
- Center for Behavioral Health Statistics and Quality (CBHSQ). 2017 National Survey on Drug Use and Health: Detailed Tables Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018
-
- CDC/NCHS, National Vital Statistics System, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC; 2018. https://wonder.cdc.gov
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

